Abstract
Introduction
Use of continuous glucose monitoring (CGM) systems by people with diabetes is associated with improved glycemic outcomes, including lower glycated hemoglobin (A1C). Less is known about adherence to CGM systems, whether glycemic outcomes are impacted by levels of adherence, or whether adherence rates differ between types of CGM systems—intermittently scanned CGM (isCGM) or real-time CGM (rtCGM).
Methods
A retrospective analysis of de-identified US administrative health claims and linked laboratory data was conducted using the Merative™ MarketScan® Research Database. The cohort included CGM-naïve people with type 1 diabetes (T1D) or type 2 diabetes treated with intensive insulin therapy (T2D-IIT) who initiated rtCGM or isCGM between August 1, 2019 and March 31, 2021 (defined as the index date). Adherence was calculated over a 12-month period using the proportion of days covered (PDC) with PDC ≥ 0.8 defined as adherent. A1C values were obtained within 6 months of the index date.
Results
A total of 7669 individuals were identified. Subgroups included T1D using isCGM (n = 1578), T1D using rtCGM (n = 1244), T2D-IIT using isCGM (n = 3567), and T2D-IIT using rtCGM (n = 1280). After 12 months, PDC was 0.71 (0.30)–0.72 (0.31) (mean(SD)) for T1D and T2D-IIT rtCGM users and 0.55 (0.34)–0.56 (0.34) for T1D and T2D-IIT isCGM users. The proportion of adherent users (PDC ≥ 0.8) was 56.8–59.7% for rtCGM users and 36.3–37.6% for isCGM users. Overall, regardless of diabetes type, the odds of adherence were over two times higher for rtCGM users compared to isCGM users. For those with available A1C information (T1D n = 213; T2D-IIT n = 346), independent of CGM type, adherence to CGM was associated with a greater reduction in A1C and more people reaching A1C targets of < 7.0% or < 8.0%.
Conclusion
For people with T1D or T2D-IIT, higher adherence to CGM is associated with greater reductions in A1C, and higher adherence rates were observed with rtCGM systems than with isCGM systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
People with type 1 diabetes (T1D) or type 2 diabetes treated with intensive insulin therapy (T2D-IIT) increasingly use continuous glucose monitoring (CGM) systems to monitor their glucose levels and manage their diabetes. |
There are two types of CGM systems—real-time CGM (rtCGM), which provides glucose values automatically to a compatible device, and intermittently scanned CGM (isCGM), which requires volitional scanning of the CGM to obtain glucose values. |
This study used medical and pharmacy payor claims and linked laboratory data to analyze adherence patterns between these two CGM systems and examined the association between CGM adherence (of any type) and changes in glycated hemoglobin (A1C). |
What was learned from the study? |
Adherence was higher among rtCGM users and, regardless of diabetes type, the odds of adherence were over twice as high for rtCGM users compared to isCGM users. |
Adherent CGM users experienced significantly greater A1C reductions compared to non-adherent CGM users. |
CGM adherence could help users with T1D and T2D-IIT lower their A1C and because rtCGM users demonstrated greater adherence, this may increase their likelihood of beneficial outcomes. |
Introduction
Continuous glucose monitoring (CGM) systems are increasingly used by people with type 1 diabetes (T1D) or type 2 diabetes using intensive insulin therapy (T2D-IIT). There are two types of CGM systems—real-time (rtCGM) and intermittently scanned (isCGM), which differ in their method of data retrieval and availability [1]. Accuracy of the latest CGM systems is quite high and some are nonadjunctive, meaning they can be used as the basis for diabetes management decisions without the use of blood glucose testing [1]. Select CGM systems can also integrate with automated insulin delivery systems such as insulin pumps or connected insulin pens [1, 2].
Use of CGM systems is associated with beneficial glycemic outcomes among people with T1D or T2D-IIT [3,4,5]. These glycemic outcomes are achieved through behavior change on behalf of the person with diabetes and careful adjustment and monitoring of their treatment regimen in consultation with a healthcare provider [6].
Adherence to diabetes medication is associated with improved glycemic control, lower healthcare utilization, reduced medical costs, and lower mortality rates [7, 8]. However, little is known about adherence to diabetes devices, such as CGM systems, and whether adherence differs between CGM types and, finally, whether device adherence is associated with glycemic improvements, such as decreased glycated hemoglobin (A1C). In this study, we queried a payor claims database to investigate adherence to CGM and analyzed whether it was associated with beneficial A1C outcomes.
Methods
Data Source
This was a retrospective, observational, US database study using de-identified administrative health claims and linked laboratory data from the Merative™ MarketScan® Research Database. The MarketScan® database is statistically de-identified and HIPAA (Health Insurance Portability and Accountability Act)-compliant and therefore the study was deemed exempt from an ethics committee review. The authors licensed the use of the database from MerativeTM. This database includes claims data for inpatient, outpatient, and outpatient prescription drug utilization of employees and their spouses and dependents or individuals with supplemental Medicare or Medicare Advantage insurance. Only data from commercially insured beneficiaries were included in the present study.
Cohort Definition
The population included adults ≥ 18 years of age with T1D or ≥ 30 years of age with T2D-IIT identified using International Classification of Diseases, 9th/10th Revisions (ICD-9/ICD-10), who were enrolled in commercial health plans (non-Medicare) and had no prior evidence of CGM use. The identification period spanned August 1, 2019 to March 31, 2021. CGM sensor initiation during this identification period was required and constituted the index date. Continuous health plan coverage 12 months pre-index (baseline) and 12 months post-index (follow-up period) was also required.
People with T2D were required to have evidence of bolus insulin (indicating intensive insulin therapy) in the baseline period. People with T1D were required to have basal and bolus or mixed insulin use at baseline. To mitigate cohort overlap by diabetes type, exclusion criteria specific to the T1D cohort included the use of basal insulin alone, sulfonylureas, thiazolidinediones (TZD), or combination drugs containing sulfonylureas or TZD during the study period. People with evidence of pregnancy or gestational diabetes were excluded.
Cohorts were segmented by CGM type using National Drug Codes (NDC) (Dexcom G5/G4 or G6 rtCGM system sensor components [8627005104 or 8627005303] or Abbott Freestyle Libre 14-day or Libre 2 isCGM system sensor components [57599000101 and 57599080000]). Individuals who had claims for a Dexcom device and an Abbott device during the identification period were excluded, making the cohorts mutually exclusive. For the subset of people included in the analysis of change in A1C, valid A1C values within 6 months before and after the index date were required.
Assessment of CGM Adherence
Adherence to CGM was calculated using the proportion of days covered (PDC). PDC is the Pharmacy Quality Alliance’s preferred metric for estimating adherence to therapies for chronic diseases such as diabetes [9]. PDC is calculated as the proportion of days in the measurement interval during which a person had access to the medication or device. Specifically, PDC is the sum of days covered by each prescription divided by the number of days from the first treated day to the end of the time period of interest. The maximum value for PDC is 1.0 and early fills or differences in CGM sensor lifetime do not impact PDC. In the full cohort, PDC was determined during the 12-month follow-up period through analysis of NDC codes related to sensor refills. PDC ≥ 0.8 was defined as adherent.
Assessment of A1C Change and Proportion Reaching Target A1C
A1C values were available for 559 out of 7669 (7.3%) of the full cohort. For inclusion in the analysis, at least one laboratory A1C value within 6 months pre- and post-index was required. Change in A1C was calculated as the mean A1C post-index minus the mean A1C pre-index during the 6-month period, with negative values indicating improvement in A1C.
In addition to assessing A1C change, the proportion reaching recommended A1C targets was also assessed. The American Diabetes Association (ADA) Standards of Care recommends an A1C goal of < 7.0% for non-pregnant adults without significant hypoglycemia [10]. The National Committee for Quality Assurance collects Healthcare Effectiveness Data and Information Set (HEDIS) survey results directly from health plans and preferred provider organizations. HEDIS collects data on multiple diabetes-related health metrics including A1C levels, with A1C control defined as < 8.0% [11, 12]. The proportion reaching these targets of < 7.0% and < 8.0% at baseline and follow-up was quantified.
Statistical Analyses
Descriptive statistics, including percentages, means, and standard deviations (SDs), were calculated for demographic characteristics and study outcomes and were presented by diabetes type (T1D or T2D-IIT) or CGM type (rtCGM or isCGM). Bivariate differences in adherence between CGM type were tested with independent sample t tests for continuous measures or Χ2 tests for categorical measures. A logit model was used to regress the dependent variable (post-index CGM adherence) on the independent variable (type of CGM) and covariates (gender, Charlson comorbidity score, insulin pump use during follow-up, and mean number of oral diabetic medications used at baseline). CGM adherence was dichotomized as PDC < 0.8 (non-adherent) or PDC ≥ 0.8 (adherent). Change in A1C between non-adherent and adherent CGM users irrespective of device type was assessed using difference-in-differences (DiD) tests. The DiD estimate indicates the magnitude and direction of change in outcome (A1C change) between the two groups. Pearson correlations assessed the association between CGM adherence and A1C change. Analyses were performed using Instant Health Data software (Panalgo, Boston, MA) and R (R Foundation for Statistical Computing, Vienna, Austria), version 3.2.1.
Results
A total of 7669 commercially insured beneficiaries with T1D or T2D treated with intensive insulin therapy (T2D-IIT) were identified. The majority were male with an average age in the low 50s (range 30–66 years) for the T2D-IIT cohort and low 40s (range 18–66 years) for the T1D cohort (Table 1).
CGM Adherence Patterns
To determine adherence rates among users of CGM systems—rtCGM or isCGM—we analyzed the PDC. After analyzing NDC codes for sensor refills over 12 months among users with T1D, the PDC was 0.72 (0.31) (mean (SD)) for rtCGM and 0.55 (0.34) for isCGM (p < 0.0001). This was comparable among users with T2D-IIT, where the PDC was 0.71 (0.30) for rtCGM sensor users and 0.56 (0.34) for isCGM sensor users (p < 0.0001).
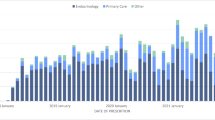
The proportion of adherent users to rtCGM was 59.7% for users with T1D and 56.8% for users with T2D-IIT (Fig. 1a). In contrast, among isCGM users with T1D or T2D-IIT, the proportion of adherent users was 36.3% or 37.6%, respectively (Fig. 1b). Overall, regardless of diabetes type, the odds of adherence were over twice as high for rtCGM users compared to isCGM users (T1D odds ratio 2.8 (95% CI 2.4–3.3), p < 0.0001; T2D-IIT odds ratio 2.2 (95% CI 1.9–2.5), p < 0.0001). In addition, the proportion of users with very low adherence (PDC < 0.25) was approximately twice as high for isCGM users compared to rtCGM users (Fig. 1).
Adherence and A1C
The association between CGM adherence and change in A1C was assessed by testing the DiD between non-adherent and adherent CGM users, regardless of type of CGM system (i.e., rtCGM or isCGM) (Table 2). Adherent CGM users achieved significantly greater A1C improvements compared to non-adherent CGM users.
Irrespective of diabetes type, adherent CGM users were more likely to reach target A1C. Among adherent users with T1D or T2D-IIT, 69.8% and 66.1% met the HEDIS target of < 8.0% at follow-up, respectively. Among non-adherent users with T1D or T2D-IIT, only 45.7% and 45.5%, respectively, met the same target (Table 3). Similarly, 33.6% and 28.6% of adherent CGM users with T1D or T2D-IIT met the ADA target of < 7.0% at follow-up, compared to 23.4% and 20.2% of non-adherent CGM users with T1D or T2D-IIT. In addition, in all cases, adherent users experienced a greater increase in the proportion achieving the A1C target, despite higher baseline proportions already meeting the target value (Table 3 and Fig. 2). Greater CGM adherence was associated with greater decreases in A1C at follow-up among users with T1D (r = − 0.16, CI [− 0.03, − 0.29], p = 0.02) or T2D-IIT (r = − 0.14, CI [− 0.03, − 0.24], p = 0.01).
Proportion meeting A1C targets. Cumulative distribution plots of A1C at baseline and follow-up among A non-adherent users with type 1 diabetes (T1D), B adherent users with T1D, C non-adherent users with type 2 diabetes treated with intensive insulin therapy (T2D-IIT), and D adherent users with T2D-IIT
Discussion
This study indicates that adherence based on PDC was significantly higher among rtCGM users compared to isCGM users in both T1D and T2D-IIT populations. In addition, greater adherence to CGM was associated with significant and clinically meaningful improvements in A1C [13]. This supports the ADA’s Standards of Care in Diabetes which recommends CGM for adults with diabetes using intensive insulin therapy to manage their diabetes [14]. Users’ higher adherence to rtCGM is relevant to the recommendation that CGM devices should be used as close to daily as possible for maximal benefit [14].
Measurement of adherence is critical to understanding treatment efficacy in the real world, but adherence metrics for durable medical devices, such as insulin pumps, can be poor indicators of their effectiveness or efficiency. CGM systems, however, do lend themselves to pharmacy refill analyses similar to pharmaceutical medications owing to their generally monthly refill schedule. Adherence to diabetes therapies is important to assess because A1C could remain high because of low treatment efficacy, low treatment adherence, or a combination of both [15]. Our analysis found an association between higher adherence to CGM systems and improved glycemic outcomes. This suggests that CGM systems can contribute to favorable and clinically meaningful outcomes, but adherence may be important to optimize glycemic control.
Our analysis found that the odds of adherence were over two times greater among users of rtCGM systems than for users of isCGM systems. This higher adherence to rtCGM could be due to feature optionality on rtCGM systems, such as a predictive hypoglycemia alert and real-time data availability without the need to physically scan the sensor. Individual motivations for using CGM could also play a role in adherence with potentially higher adherence among rtCGM users interested in preventing severe hypoglycemic or hyperglycemic events. In addition, differences in insurance coverage or out-of-pocket costs could also contribute to adherence patterns. About twice as many isCGM users had very low adherence (PDC < 0.25), compared to rtCGM, indicating higher rates of sporadic or short-term use.
This lower adherence among isCGM users compared to rtCGM users is notable because CGM use is associated with reductions in A1C and a lower risk of acute and long-term diabetes complications [3, 4, 16,17,18,19]. It is unknown whether people using insulin who stopped using CGM returned to self-monitoring of blood glucose (SMBG) regimens or administered insulin with little to no information about their glucose levels.
Our results found a greater decrease in A1C among people with diabetes who were more adherent to CGM. Beyond the individual benefits of lowering A1C [16, 19,20,21,22], improved population-level outcomes are important to healthcare payors. A recent analysis of the cost-effectiveness of rtCGM vs. isCGM among people with T2D using multiple daily injections of insulin (a subset of T2D-IIT) from a US payor perspective found rtCGM to be cost-saving over isCGM [23]. The larger A1C decreases, on average, among adherent CGM users may further contribute to increased cost-effectiveness in this population. However, it is also important to note that while about two-thirds of those adherent to CGM met the HEDIS target of A1C < 8.0%, only about one-third met the ADA target of A1C < 7.0%. Helping more people with diabetes who use insulin reach the ADA recommendation may require increasing availability of diabetes education programs and use of newer technologies such as hybrid closed-loop systems.
Limitations of this study include the all-US cohort, the lack of racial and ethnic data, and the relatively low proportion of the cohort with A1C data. The findings may not be generalizable beyond people with commercial insurance coverage in the USA. An additional limitation is that this is a retrospective data analysis. Medication changes over the 6-month observation window could have impacted the observed A1C results. Adherence was assessed through calculation of PDC, which is an indirect estimate of adherence obtained from pharmacy refill data. Actual utilization could differ from observed utilization and the degree to which people used the CGM devices to monitor their glucose levels and change their behavior cannot be assessed. Our analysis removed people with a pharmacy claim for more than one type of CGM system and thus we did not assess patterns of switching between systems. The analysis did not include extensive assessment of the use of insulin pumps and hybrid closed-loop systems beyond statistical adjustment for pump use in our multivariate model. Additional research is needed to assess CGM adherence in relation to other diabetes technologies. As new CGM devices, with new features and functionality, enter the market, their adherence will have to be assessed and the current findings may not generalize to these devices.
Conclusions
Our findings indicate that for people with T1D or T2D-IIT, higher adherence to CGM could help them lower their A1C. RtCGM users were also significantly more likely to be adherent to CGM, potentially increasing the likelihood of beneficial outcomes. Because of CGM’s association with A1C improvements and fewer acute and long-term complications, it is expected that high CGM adherence could further improve outcomes and lower individual and payor healthcare costs.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to confidentiality.
References
Friedman JG, Cardona Matos Z, Szmuilowicz ED, Aleppo G. Use of continuous glucose monitors to manage type 1 diabetes mellitus: progress, challenges, and recommendations. Pharmgenomics Pers Med. 2023;16:263–76. https://doi.org/10.2147/PGPM.S374663.
Sangave NA, Aungst TD, Patel DK. Smart connected insulin pens, caps, and attachments: a review of the future of diabetes technology. Diabetes Spectr. 2019;32(4):378–84. https://doi.org/10.2337/ds18-0069.
Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371–8. https://doi.org/10.1001/jama.2016.19975.
Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–74. https://doi.org/10.7326/M16-2855.
Mustonen J, Rautiainen P, Lamidi ML, Lavikainen P, Martikainen J, Laatikainen T. Marked improvement in A1C levels after initiation of intermittently scanned continuous glucose monitoring is maintained over 4 years in patients with type 1 diabetes. Diabetes Spectr. 2022;35(4):469–75. https://doi.org/10.2337/ds21-0087.
American Diabetes Association Professional Practice Committee. 5. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes—2024. Diabetes Care. 2023;47(Supplement_1):S77–110. https://doi.org/10.2337/dc24-S005.
Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299–307. https://doi.org/10.2147/PPA.S106821.
Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33(1):74–109. https://doi.org/10.1016/j.clinthera.2011.01.019.
Pharmacy Quality Alliance. PQA measure overview. 2022. https://www.pqaalliance.org/assets/Measures/PQA_Measures_Overview.pdf. Accessed 16 Aug 2023
American Diabetes Association Professional Practice Committee. 6 glycemic goals and hypoglycemia: standards of care in diabetes—2024. Diabetes Care. 2023;47(Supplement_1):S111–25. https://doi.org/10.2337/dc24-S006.
National Committee for Quality Assurance. Comprehensive Diabetes Care (CDC). 2023. https://www.ncqa.org/hedis/measures/comprehensive-diabetes-care/. Accessed 4 Oct 2023
U.S. Department of Health and Human Services. Healthcare Effectiveness Data and Information Set (HEDIS). 2023. https://health.gov/healthypeople/objectives-and-data/data-sources-and-methods/data-sources/healthcare-effectiveness-data-and-information-set-hedis. Accessed 4 Oct 2023
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603. https://doi.org/10.2337/dci19-0028.
American Diabetes Association Professional Practice Committee. 7. Diabetes Technology: Standards of Care in Diabetes—2024. Diabetes Care. 2023;47(Supplement_1):S126–44. https://doi.org/10.2337/dc24-S007.
Johnson LM, Swarner SL, van der Straten A, Rothrock GD. In: Methods for assessing the adherence to medical devices. Research Triangle Park: RTI; 2016.
Reaven PD, Newell M, Rivas S, Zhou X, Norman GJ, Zhou JJ. Initiation of continuous glucose monitoring is linked to improved glycemic control and fewer clinical events in type 1 and type 2 diabetes in the Veterans Health Administration. Diabetes Care. 2023;46(4):854–63. https://doi.org/10.2337/dc22-2189.
Guerci B, Levrat-Guillen F, Vicaut E, et al. Reduced acute diabetes events after freestyle Libre system initiation in people 65 years or older with type 2 diabetes on intensive insulin therapy in France. Diabetes Technol Ther. 2023;25(6):384–94. https://doi.org/10.1089/dia.2023.0034.
Charleer S, De Block C, Bolsens N, et al. Sustained impact of intermittently scanned continuous glucose monitoring on treatment satisfaction and severe hypoglycemia in adults with type 1 diabetes (FUTURE): an analysis in people with normal and impaired awareness of hypoglycemia. Diabetes Technol Ther. 2023;25(4):231–41. https://doi.org/10.1089/dia.2022.0452.
Riveline JP, Roussel R, Vicaut E, et al. Reduced rate of acute diabetes events with flash glucose monitoring is sustained for 2 years after initiation: extended outcomes from the RELIEF Study. Diabetes Technol Ther. 2022;24(9):611–8. https://doi.org/10.1089/dia.2022.0085.
DCCT/EDIC Research Group. The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45(10):1289–98.
Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39(5):686–93. https://doi.org/10.2337/dc15-1990.
Guerci B, Roussel R, Levrat-Guillen F, et al. Important decrease in hospitalizations for acute diabetes events following freestyle Libre system initiation in people with type 2 diabetes on basal insulin therapy in France. Diabetes Technol Ther. 2023;25(1):20–30. https://doi.org/10.1089/dia.2022.0271.
Alshannaq H, Norman GJ, Lynch PM. 141-LB: cost-effectiveness of real-time continuous glucose monitoring (rt-CGM) vs. intermittent-scanning continuous glucose monitoring (is-CGM) from a U.S. payer perspective in patients with type 2 diabetes on multiple daily injections of insulin (PwT2D on MDI). Diabetes. 2023. https://doi.org/10.2337/db23-141-LB.
Acknowledgements
Medical Writing/Editorial Assistance
No external medical writing or editorial assistance was received during the writing of this article.
Funding
Dexcom, Inc. provided funding for this analysis and the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Poorva M. Nemlekar, Katia L. Hannah, and Gregory J. Norman were involved in conceptualization and statistical design. Poorva M. Nemlekar performed the data analyses. Katia L. Hannah validated the analyses. All authors were involved in data interpretation. Courtney R. Green drafted the manuscript. All authors provided edits to the manuscript and reviewed and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
Poorva M. Nemlekar, Katia L. Hannah, Courtney R. Green, and Gregory J. Norman are employees of Dexcom, Inc.
Ethical Approval
This retrospective observational study used only existing anonymized electronic medical record and billing data licensed by the authors from Merative™. The MarketScan® database is statistically de-identified and HIPAA-compliant and therefore the study was deemed exempt from an ethics committee review. No intervention was implemented for the purpose of the study, and no patient-identifiable information was used in the study. The study was compliant with the Helsinki Declaration of 1964, and its later amendments.
Additional information
Prior Presentation: Portions of this article were presented at the 83rd Scientific Sessions of the American Diabetes Association held in San Diego, CA on June 23–26, 2023.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nemlekar, P.M., Hannah, K.L., Green, C.R. et al. Association Between Adherence, A1C Improvement, and Type of Continuous Glucose Monitoring System in People with Type 1 Diabetes or Type 2 Diabetes Treated with Intensive Insulin Therapy. Diabetes Ther 15, 639–648 (2024). https://doi.org/10.1007/s13300-023-01529-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01529-8