Abstract
Introduction
We evaluated the effectiveness and safety of sodium-glucose cotransporter 2 inhibitor (SGLT2i) add-on treatment in patients with type 2 diabetes mellitus (T2DM) in the real-world setting.
Methods
This single-center retrospective study used the clinical database of Seoul National University Hospital in South Korea. Patients who received metformin monotherapy or combination therapy with ≥ 1 other oral hypoglycemic medication and had a baseline glycosylated hemoglobin (HbA1c) between 7.0% and 10.5% were included. Propensity score matching was applied between patients treated with and without SGLT2 inhibitors (SGLT2i and non-SGLT2i groups, respectively). Changes in HbA1c from baseline to week 26 were compared between the SGLT2i and non-SGLT2i groups, and risk of adverse events (AE) were also assessed.
Results
A total of 1106 patients were included. At week 26, HbA1c was significantly more reduced by 0.35 percentage points in the SGLT2i group than in the non-SGLT2i group (95% CI 0.30–0.41, P < 0.001). Likewise, the proportion of patients achieving HbA1c < 7% was also significantly higher (51.9% vs. 37.6%, P < 0.05) in the SGLT2i group than in the non-SGLT2i group. The risk of adverse events in the SGLT2i group was mostly comparable with those in the non-SGLT2i group except for diseases of the liver, pain, hypertensive diseases, and metabolic disorders, which showed significantly higher odds in the SGLT2i group.
Conclusions
SGLT2i add-on treatment is an effective and safe therapeutic option for patients with T2DM in the real-world practice setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The added benefit of sodium-glucose cotransporter 2 inhibitors (SGLT2i) in combination with other oral hypoglycemic medications has been demonstrated in multiple randomized controlled trials. |
We evaluated the effectiveness and safety of adding SGLT2i to metformin, sulfonylureas, and dipeptidyl peptidase 4 inhibitor combination therapy in patients with type 2 diabetes mellitus (T2DM) in a real-world setting. |
What was learned from the study? |
Our study confirmed the additional glucose-lowering benefits of SGLT2i add-on treatments in patients with T2DM in real-world settings, regardless of baseline drug combinations. |
This finding further strengthens the existing evidence supporting the add-on treatments of SGLT2i in real-world clinical practice. |
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive chronic disease that requires changes in treatment strategies to maintain glycemic targets for a long time [1]. Many clinical practice guidelines (CPGs) for T2DM recommend metformin as an initial pharmacologic treatment, followed by stepwise addition of other medications [1,2,3,4]. Four classes of oral hypoglycemic medications (OHMs) are commonly prescribed as a monotherapy or combination treatment with metformin in T2DM: sodium-glucose cotransporter 2 inhibitors (SGLT2i), dipeptidyl peptidase 4 inhibitors (DPP4i), thiazolidinediones (TZD), and sulfonylureas (SU) [2].
SGLT2i have a unique glucose-lowering action by increasing the urinary excretion of glucose [5]. SGLT2i not only improve glycemic control with no increased risk of hypoglycemia but they also reduce body weight and blood pressure [6,7,8]. In addition, SGLT2i significantly decreased major adverse cardiovascular events and mortality outcomes in patients with T2DM [9,10,11,12,13,14]. Furthermore, because the mode of action of SGLT2i is distinct from other OHMs that preserve β-cell function or enhance insulin sensitivity, combination of SGLT2i with other OHMs has shown additional efficacy while not compromising safety [6].
Although the added benefit of SGLT2i in combination with other OHMs has been demonstrated in multiple randomized controlled trials (RCT), the comparative effectiveness of combination therapy with and without SGLT2i has not been established in a real-world setting. Several observational studies showed that adding or switching to SGLT2i significantly reduced HbA1c level from baseline [15, 16]. However, to the best of our knowledge, no research has been done to evaluate if SGLT2i add-on therapy is more effective in glycemic control than treatments without SGLT2i in various combination regimens that are frequently prescribed in real-world clinical practice.
In this study, we aimed to evaluate the effectiveness and safety of SGLT2i as an additional treatment for patients with T2DM in the real-world practice setting. To this end, we compared the glycemic control and risk of adverse events in patients with T2DM treated with or without SGTL2i added to MET, SU, and DPP4i using real-world data.
Methods
Data Source
This single-center retrospective observational study used an electronic medical record database at Seoul National University Hospital (SNUH), extracted, transformed, and loaded according to the specifications of the Observational Medical Outcomes Partnership Common Data Model (version 5.3.1, OMOP CDM). SNUH is a university-affiliated, tertiary-care hospital in Seoul, South Korea. We extracted data on demographics, conditions, drug exposures, procedures, measurements, and hospital visits using de-identified patient records in SNUH CDM from October 2004 to June 2021.
Study Design and Population
Eligible subjects were patients with T2DM who were ≥ 19 years old, received MET monotherapy or combination therapy of MET and ≥ 1 other OHM(s) among SU, DPP4i, and SGLT2i for ≥ 183 consecutive days with no change in the treatment regimen and ≤ 3 days of drug holiday, and had a baseline glycosylated hemoglobin (HbA1c) of 7.0–10.5%, both inclusive. Patients were excluded if they met any of the following conditions: (1) patients with type 1 diabetes, (2) pregnant patients, (3) patients who were prescribed antidiabetic medications other than MET, SU, DPP4i, and SGLT2i (e.g., TZD, α-glucosidase inhibitors, insulin, glucagon-like peptide 1) during the baseline period (i.e., within 120 days before the index date), (4) patients who did not have HbA1c records at baseline and the follow-up (i.e., within 120 days after week 26) period.
The study population was divided into four cohorts by treatment: MET with or without SGLT2i (cohort 1), MET and SU with or without SGLT2i (cohort 2), MET and DPP4i with or without SGLT2i (cohort 3), and MET, SU, and DPP4i with or without or SGLT2i (cohort 4). A fixed-dose combination (FDC) drug was considered the same as the combination of individual drugs constituting the combination.
Outcomes
We compared changes in HbA1c from baseline to week 26 between patients treated with and without add-on SLGT2i in each cohort. For those treated with SGLT2i (SGLT2i group), the index date was the first prescription day of SGLT2i as an add-on treatment. For those who were not treated with SGLT2i (non-SGLT2i group), the index date was the first day of prescription that had been maintained for ≥ 183 days without regimen change. The baseline HbA1c was the one measured on the index date or the previous closest date to the index date. Likewise, the follow-up HbA1c was the one measured at week 26 or on the next closest date within 120 days.
We defined an adverse event (AE) as a diagnosis, sign, or symptom of a condition newly observed in patients after the index date. AEs that occurred in more than 1% of patients were classified using the 2021 ICD-10-CM codes, which describe the general type of injury or disease. For safety assessment, the risk of each adverse event was compared between the SGLT2i and non-SGLT2i groups.
Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation, and categorical variables as numbers and percentages. Continuous and categorical variables were compared between the SGLT2i and non-SGLT2i groups by using the Student’s t test and Pearson’s chi-square test, respectively. To balance the baseline characteristics between the SGLT2i and non-SGLT2i groups, we performed propensity score matching with a 1:1 ratio and the nearest neighbor matching method [17] in each cohort using the MatchIt package in R version 4.1.3 (R foundation, Vienna, Austria). A propensity score was estimated by a multiple logistic regression model adjusted for covariates such as age, sex, baseline HbA1c, concomitant use of aspirin, cardiovascular drugs, antidyslipidemic drugs, antihypertensive drugs, ophthalmic drugs, and comorbidity with cardiovascular disease, dyslipidemia, hypertension, metabolic disease, renal disease, and diabetic retinopathy. A comparison of changes in HbA1c between the SGLT2i and non-SGLT2i groups was performed using analysis of covariance, where age, sex, baseline HbA1c, concomitant medications, and comorbidities were the covariates. The changes in HbA1c were presented as least-squares (LS) mean and standard deviation. In safety analysis, we compared AE occurrence after the index date between the SGLT2i and non-SGLT2i groups. The odds ratio (OR) was used to assess the risk of adverse events. All statistical analyses were performed using R version 4.1.3 (R foundation, Vienna, Austria). A two-sided P value < 0.05 was considered statistically significant.
Ethics Statement
This study was approved by the SNUH Institutional Review Board (IRB No. H-2003-013-1106). As this was a retrospective study using de-identified data source, obtaining informed consent was waived by the SNUH IRB.
Results
Study Population and Baseline Characteristics
Of 94,208 initially screened patients with T2DM, 1106 patients were included in the analysis (Fig. 1). After propensity score matching, demographics and clinical characteristics became balanced between the SGLT2i and non-SGLT2i groups (Table 1). The mean age was 58 and 59 years in the SGLT2i and the non-SGLT2i groups, respectively. In both groups, approximately 63% patients were male, and the mean baseline HbA1c was 8.1% (Table 1). In each cohort, the demographics and clinical characteristics of the two groups were comparable (Table S1 in the supplementary material).
Effectiveness of SGLT2i Add-on Treatment
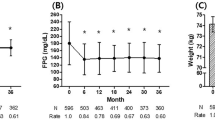
At week 26, the SGLT2i group showed a significantly greater reduction in HbA1c from baseline than the non-SGLT2i group (− 0.35 percentage points, 95% CI − 0.41 to − 0.30, P < 0.001) while reduction in HbA1c from baseline to week 26 was statistically significant in both groups (Fig. 2). In all of the cohorts, the decreases in HbA1C were larger in the SGLT2i group than in the non-SGLT2i group. The differences in HbA1c change between the two groups in each cohort ranged from − 0.46 percentage points (95% CI − 0.58 to − 0.34, P < 0.018) to − 0.22 percentage points (95% CI − 0.35 to − 0.09, P < 0.092) (Fig. 2). In addition, the proportion of patients achieving HbA1c < 7% was significantly higher in patients treated with SGLT2i as add-on treatment at week 26 (51.9% in the SGLT2i and 37.6% in the non-SGLT2i groups, respectively, P < 0.05) (Fig. 3). The individual drugs that the patients received in all combinations are summarized in Table S2 in the supplementary material.
Changes in glycosylated hemoglobin after 26 weeks of treatment. LS least square, CI confidence interval, HbA1c glycosylated hemoglobin, SGLT2i sodium-glucose cotransporter 2 inhibitor, MET metformin, SU sulfonylureas, DPP4i dipeptidyl peptidase 4 inhibitor. aP < 0.05 for HbA1c from baseline to week 26 by the paired t test. bHbA1c in the SGLT2i group minus the one in the non-SGLT2i group. cP value for difference between the non-SGLT2i and SGLT2i groups by the analysis of covariance
Safety of SGLT2i Add-on Treatment
The odds of diseases of the liver (OR 2.96; 95% CI 1.81–4.85), hypertensive diseases (OR 2.33; 95% CI 1.30–4.17), pain (OR 1.97; 95% CI 1.28–3.03), and metabolic disorders (OR 1.90; 95% CI 1.20–2.99) were significantly higher in the SGLT2i group than in the non-SGLT2i group (Fig. 4). In contrast, the SGLT2i group had a significantly lower occurrence of disease of male genital organs than the non-SGLT2i group (OR 0.45; 95% CI 0.23–0.88) (Fig. 4). There was no significant difference in the risk of other AEs between the two groups.
Odds ratio for adverse events in the SGLT2i and non-SGLT2i group. SGLT2i sodium-glucose cotransporter 2 inhibitor, CI confidence interval. Values are presented as number (%). aAdverse event was categorized on the basis of 2021 ICD-10-CM category. bP value for homogeneity of odds between the two groups by chi-square test
Discussion
We showed that SGLT2i add-on treatments had additional glucose-lowering benefit in patients with T2DM in the real world regardless of baseline drug combinations. HbA1c was significantly more reduced by 0.35 percentage points (95% CI 0.30–0.41, P < 0.001, Fig. 2) in patients treated with SGLT2i than in those not treated with SGLT2i. In addition, the percentage of patients who attained the glycemic target (i.e., HbA1c < 7%) was significantly higher at week 26 in patients receiving SGLT2i than in those not receiving SGLT2i (51.9% and 37.6%, respectively; P < 0.05, Fig. 3). Although some adverse events such as diseases of the liver, hypertensive diseases, pain, and metabolic disorders occurred more frequently in the SGLT2i group than in the non-SGLT2i group, the majority of adverse events occurred in fewer than 10% of patients (Fig. 4).
Although SGLT2i decreased HbA1c further, the degree of reduction in this study was relatively lower than that found in RCTs. For example, RCTs reported that the adjusted mean difference in change in HbA1c ranged from 0.64 to 0.77 percentage points, being greater in patients treated with MET and SGLT2i than in those treated with MET alone, while it was 0.45 percentage points in the present study (Fig. 2) [18, 19]. Likewise, the adjusted mean decrease in HbA1c in patients treated with SGLT2 were 0.72–0.9 percentage points greater in patients treated with triple combination therapy of MET, DPP4i, and SGLT2i than those treated with dual combination therapy of MET and DPP4, whereas it was 0.46 percentage points in the present study (Fig. 2) [20, 21]. Those previous examples show that the treatment effects were generally higher in RCTs than in the real world probably because RCTs are conducted in a highly controlled environment with a homogeneous and limited population [22]. Therefore, direct extrapolation of the results of RCTs to diverse real-world scenarios can be challenging. However, it is noteworthy that the additional glucose-lowering effect of SGLT2i shown in previous RCTs was repeated in the real world as the present study clearly demonstrated.
Some divergent findings were noted in the safety of SGLT2i in this study from those reported in RCTs. First, hypoglycemia, genital infection (GTI), and urinary tract infection (UTI), commonly reported AEs in patients receiving SGLT2i in RCTs [23, 24], were not observed in this study. It was probably because our data did not include the laboratory measurements that could suggest hypoglycemia, GTI, or UTI. Furthermore, because GTI and UTI are associated with relatively minor symptoms, patients with GTI and UTI might have sought treatment at nearby primary care clinics rather than scheduling and waiting for tertiary hospital visits. Second, recent studies have reported that SGLT2i reduced fatty liver content and improved biomarkers of non-alcoholic fatty liver disease (NAFLD) in patients with T2DM [25]. In this study, a significantly higher risk of liver disease was noted in the SGLT2i group than in the non-SGLT2i group (OR 2.96; 95% CI 1.81–4.85, Fig. 4). Of the diseases of the liver seen in the present study, only one case of NAFLD was observed in the non-SGLT group, whereas the most frequent sub-disease was alcoholic fatty liver and abnormal test results. The occurrence of alcoholic fatty liver (3.8% in the SGLT2i group and 1.3% in the non-SGLT2i group, respectively, Table S3 in the supplementary material) and abnormal test results (6.1% in the SGLT2i group and 2.2% in the non-SGLT2i group, respectively, Table S3 in the supplementary material) were higher in the SGLT2i group than in the non-SGLT2 group. However, the association between these two conditions and SGLT2i has yet to be established.
A surprising finding was that the occurrence of benign prostatic hyperplasia (BPH), which accounts for 95.1% among sub-diseases classified as the disease of male genital organs, was two-fold lower in the SGLT group than in the non-SGLT2i group (2.4% in the SGLT2i group and 4.7% in the non-SGLT2i group, respectively, Table S3 in the supplementary material). Risk factors affecting the development of BPH include vascular damage, atherosclerosis, and dyslipidemia [26,27,28]. Notably, SGLT2i have been demonstrated to improve endothelial function and lipid profile, in turn reducing arterial stiffness and the risk of major adverse cardiovascular events [29, 30]. Thus, the lower occurrence of BPH in the SGLT2i group may be associated with the beneficial effects of SGLT2is on the risk factors for developing BPH.
This study had several limitations. First, the duration of diabetes in patients was not considered because the first diagnosis date of patients was not identifiable particularly for those who were diagnosed with T2DM outside of the Seoul National University Hospital. A longer duration of diabetes may indicate higher disease severity and a greater risk of diabetes complications [31, 32]. To balance the severity of diabetes, we adjusted the baseline HbA1c between the two groups using propensity score matching. Second, although all baseline characteristics became comparable through propensity score matching, there was still a possibility that some covariates, particularly for those unmeasured, did not distribute equally between the SGLT2i and non-SGLT2i groups. This could have affected the association between a specific covariate and the change in HbA1c. To overcome this limitation, we adjusted for the covariates using multiple logistic regression analysis. Lastly, although this study analyzed large-scale data consisting of 1106 patients, it is limited in that it used data from a single institution. To validate and confirm the effectiveness and safety of additional combination therapy with SGLT2 inhibitors across diverse clinical settings, further multicenter studies are warranted.
Conclusion
Our study evaluated the additive effectiveness and safety of SGLT2i add-on treatment in patients treated with multiple OHM class combinations commonly prescribed in the real world. In conclusion, SGLT2i add-on treatment is an effective and safe therapeutic option for patients with T2DM in the real-world practice setting.
Data Availability
The dataset generated and analyzed for the current study are not publicly available because they are privately owned by Seoul National University Hospital.
References
Committee American Diabetes Association Professional Practice. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes–2022. Diabetes Care. 2021;45:S125–43.
Committee of Clinical Practice Guidelines KDA. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019;43(4):398–406.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
IDF Working group. IDF clinical practice recommendations for managing type 2 diabetes in primary care. Brussels: International Diabetes Federation. 2017. ISBN: 978-2-930229-85-0.
Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context. 2014;3: 212264.
Van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, Jzerman RGI, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41(8):1543–56.
Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18(8):783–94.
Qiu M, Zhao LM, Zhan ZL. Comprehensive analysis of adverse events associated with SGLT2is: a meta-analysis involving nine large randomized trials. Front Endocrinol (Lausanne). 2021;12: 743807.
Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (canagliflozin cardiovascular assessment study). Circulation. 2018;137(4):323–34.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Neal B, Perkovic V, Matthews DR, et al. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19(3):387–93.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Hong AR, Koo BK, Kim SW, Yi KH, Moon MK. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in Korean patients with type 2 diabetes mellitus in real-world clinical practice. Diabetes Metab J. 2019;43(5):590–606.
Ertugrul DT, Kan E, Tura CB, et al. Add-on therapy with dapagliflozin in routine outpatient care of type 2 diabetes patients from Turkey: a retrospective cohort study on HbA1c, body weight, and blood pressure outcomes. Int J Diabetes Develop Countries. 2021;42(1):147–60.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396–404.
Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582–92.
Mathieu C, Ranetti AE, Li D, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38(11):2009–17.
Rodbard HW, Seufert J, Aggarwal N, et al. Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin. Diabetes Obes Metab. 2016;18(8):812–9.
Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33(34):e213.
Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824.
Qiu M, Ding LL, Zhang M, Zhou HR. Safety of four SGLT2 inhibitors in three chronic diseases: a meta-analysis of large randomized trials of SGLT2 inhibitors. Diab Vasc Dis Res. 2021;18(2):14791641211011016.
Scheen AJ. Beneficial effects of SGLT2 inhibitors on fatty liver in type 2 diabetes: a common comorbidity associated with severe complications. Diabetes Metab. 2019;45(3):213–23.
Berger AP, Bartsch G, Deibl M, et al. Atherosclerosis as a risk factor for benign prostatic hyperplasia. BJU Int. 2006;98(5):1038–42.
Berger AP, Deibl M, Leonhartsberger N, et al. Vascular damage as a risk factor for benign prostatic hyperplasia and erectile dysfunction. BJU Int. 2005;96(7):1073–8.
Gacci M, Sebastianelli A, Salvi M, et al. Benign prostatic enlargement can be influenced by metabolic profile: results of a multicenter prospective study. BMC Urol. 2017;17(1):22.
Soares RN, Ramirez-Perez FI, Cabral-Amador FJ, et al. SGLT2 inhibition attenuates arterial dysfunction and decreases vascular F-actin content and expression of proteins associated with oxidative stress in aged mice. Geroscience. 2022;44(3):1657–75.
McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–58.
Juarez DT, Sentell T, Tokumaru S, Goo R, Davis JW, Mau MM. Factors associated with poor glycemic control or wide glycemic variability among diabetes patients in Hawaii, 2006–2009. Prev Chronic Dis. 2012;9: 120065.
Hayashino Y, Izumi K, Okamura S, Nishimura R, Origasa H, Tajima N, group Js. Duration of diabetes and types of diabetes therapy in Japanese patients with type 2 diabetes: The Japan Diabetes Complication and its Prevention prospective study 3 (JDCP study 3). J Diabetes Investig. 2017;8(2):243–9.
Acknowledgements
We thank Suyeon Hong for reviewing clinical data.
Funding
This research and the journal’s Rapid Service Fee was supported by the BK21FOUR Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (5120200513755). The study was supported by a grant of the Seoul National University Hospital (0620181130).
Author information
Authors and Affiliations
Contributions
Conceptualization: Yesol Hong, Yoomin Jeon, Howard Lee. Methodology: Yesol Hong, Yoomin Jeon, Yoona Choi, Tae Kyu Chung, Howard Lee. Formal analysis and investigation: Yesol Hong, Tae Kyu Chung, Howard Lee. Writing-original draft preparation: Yesol Hong, Yoona Choi, Howard Lee. Writing-review and editing: Yesol Hong, Howard Lee.
Corresponding author
Ethics declarations
Conflict of Interest
Yesol Hong, Yoomin Jeon, Yoona Choi, Tae Kyu Chung, and Howard Lee have no conflict of interest to disclose.
Ethical Approval
This study was approved by the SNUH Institutional Review Board (IRB No. H-2003-013-1106). As this was a retrospective study using de-identified data source, obtaining informed consent was waived by the SNUH IRB.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hong, Y., Jeon, Y., Choi, Y. et al. Effectiveness and Safety of Sodium-Glucose Cotransporter 2 Inhibitors Added to Dual or Triple Treatment in Patients with Type 2 Diabetes Mellitus. Diabetes Ther 15, 487–496 (2024). https://doi.org/10.1007/s13300-023-01518-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01518-x