Abstract
Introduction
This study aimed to assess the safety and effectiveness of semaglutide, administered either by weekly subcutaneous (SC) injection or orally, in real-life practice in Saudi Arabia in individuals with type 2 diabetes mellitus (T2DM).
Methods
A retrospective chart review study was conducted at 18 Saudi Arabia centers. An accredited centralized institutional review board approved the study. Medical records were included for individuals of any age ≥ 18 years with uncontrolled T2DM. The primary outcome measure was the laboratory glycated hemoglobin (HbA1c) level. Secondary measures included fasting blood glucose (FBG), weight, and hypoglycemia. All variables were checked after 6 and 12 months of semaglutide initiation.
Results
The analysis of this study included 1223 patients with uncontrolled T2DM (HbA1c > 7%). The mean (SD) baseline HbA1c was 10.02% (1.17). HbA1c was reduced by an average of 3.02% (0.84) and 3.17% (0.84) at 6 and 12 months, respectively. Results of a repeated measure analysis of variance (ANOVA) indicated significant differences in HbA1c (p value < 0.001). HbA1c levels at 6 and 12 months were significantly lower, 7.00% (0.70) and 6.85% (0.69), than at baseline, 10.02% (1.17). About 193 patients (56.4%) of the 295 patients having HbA1c < 9% achieved HbA1c of 5.7% or less. The frequency of hypoglycemia events was 4.60 (1.10) in the 3 months before semaglutide was initiated. The frequency of hypoglycemia events in the last 3 months was 2.30 (0.80) events and 0.80 (0.50) events at 6-month and 12-month follow-up visits, respectively.
The percent reduction in body mass index (BMI) was an average of 13.07% (1.53) and 19.89% (4.07) at 6 and 12 months, respectively. Lipid profile and blood pressure were improved at 6 and 12 months.
Conclusion
Semaglutide, administered either by SC injection or orally, provided substantial glycemic and weight-loss benefits in adults with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The burden of diabetes is a significant global issue and particularly in Saudi Arabia (KSA) where the trend of diabetes is escalating. |
When a new medication for diabetes like the human glucagon-like peptide 1 (GLP-1) analogue semaglutide, with its established efficacy and safety for glycemic control and weight loss, is supplied under typical clinical practice situations, real-world evidence is increasingly used to supplement the data from randomized clinical trials and assess the efficacy and safety of those medications in the local setting and in real-life practice. |
This real-world study aimed to assess the safety and effectiveness of semaglutide in individuals with uncontrolled T2DM in Saudi Arabia. The primary outcome measure was the laboratory HbA1c level. Secondary measures included FBG, weight, and hypoglycemia. All variables were checked 6 and 12 months after semaglutide initiation. |
What was learned from the study? |
The analysis of this study included 1223 patients. The study showed a significant reduction in HbA1c after semaglutide initiation. Also, significant weight reduction was demonstrated. In addition, the frequency of hypoglycemia events was comparable. There were few adverse events reported and these disappeared with time. |
In conclusion, semaglutide, administered either by SC injection or orally, provided substantial glycemic and weight-loss benefits in adults with T2DM and was a safe option for them. |
The current study confirmed the initial hypothesis and proved that the utilization of semaglutide in patients with uncontrolled diabetes in a real-world setting can be a good asset for those people. |
We recommend further well-designed randomized controlled trials (RCTs) in the local setting. |
Introduction
The burden of diabetes mellitus (DM) is a significant global issue. Attention has been brought to this health issue, particularly in Saudi Arabia (KSA), one of the top ten countries with the highest DM prevalence (estimated to rank among the top five in 2030) [1]. According to Al Nozah et al., the prevalence of DM in KSA between 1995 and 2000 was 24%, a tenfold rise from 2.5% in 1982 [1,2,3]. Additionally, KSA had 4.3 million persons with DM in 2019, the fourth-highest among MENA countries (Middle East and North Africa). Further, the estimated age-adjusted prevalence of DM in KSA was 15.8%, costing USD 5,012,600. According to projections [4,5,6,7], the prevalence of DM will be 17.2% in 2030 and 17.8% in 2045.
Semaglutide, a human glucagon-like peptide 1 (GLP-1) analogue and GLP-1 receptor agonist (GLP-1RA) with a lengthy half-life, can be administered once weekly (OW) [8]. The SUSTAIN program of randomized control trials (RCT) established the efficacy and safety of semaglutide, showing superior and clinically significant glycemic control and weight loss compared with placebo or active comparators in a wide range of subjects with T2DM as an add-on to various oral glucose-lowering medications, including in combination with sodium-glucose cotransporter 2 inhibitors (SGLT2is) [9,10,11,12,13,14,15,16,17,18].
When new medications are supplied under typical clinical practice situations, real-world evidence (RWE) is increasingly used to supplement the data from randomized clinical trials and assess the efficacy and safety of those medications [19, 20].
Regulatory agencies in KSA, such as the Saudi Food and Drug Administration (SFDA), have approved semaglutide as a supplement to diet and exercise for treating adults with T2DM that has not been satisfactorily controlled. The most efficient strategy to avoid and lessen the burden of T2DM is to achieve the desired glycemic control targets, especially HbA1c. Unfortunately, reaching glycemic targets is very difficult in most patients with DM.
This retrospective study’s goal was to provide information on the practical application and effects of semaglutide in Saudi patients with T2DM who were GLP-1RA naïve or switchers under routine medical supervision. The characteristics of patients were documented to phenotype the semaglutide-treated population. Therefore, the study evaluated changes in HbA1c, weight, important clinical parameters, discontinuation rate, and safety in both GLP-1RA-naïve patients and those switching from another GLP-1RA at 6 and 12 months following semaglutide initiation.
Methods
The current multicenter, observational, retrospective, cohort, single-arm, chart review study was carried out at 18 centers in KSA (12 centers with eligible data for analysis, 6 centers data was not suitable for analysis; Supplementary Material). Each center contributed 48–178 patients to the analysis, with an average of 102 (SD = 47) patients per center.
The study conformed to the principles of the 2011 Declaration of Helsinki and the Good Pharmacoepidemiology Practices (GPP) guidelines. An accredited centralized institutional review board (IRB) approved the study, and informed consent was waived by the IRB as the study was retrospective.
The 18 centers each conducted a database search for potential patients’ records from April 2020 until April 2023 to be included in the study. The charts, including the medical records of individuals of any age with T2DM treated with diet, insulin therapy, orally available glucose-lowering medications, or non-insulin injection therapy, were included. The data taken from the charts was Health Insurance Portability and Accountability Act (HIPAA) de-identified (anonymized) in compliance with the HIPAA Privacy Rule [21].
Individuals 18 years of age or more and of any gender should have their HbA1c readings documented for the most recent 3–6 months before starting semaglutide to be included in the analysis of this study. This study’s analysis did not include subjects with controlled DM (HbA1c ≤ 7).
At the start, we planned the study to be from 18 centers; however, the analysis included complete data from only 12 centers as data from six centers was incomplete. For a record to be included in the analysis, all data should be complete without any missing values, particularly for the glycemic control outcome variables (HbA1c and BG) and weight reduction.
All of the following subjects were considered for the statistical analysis: men or women, age of 18 years or more, with a diagnosis of T2DM according to the American Diabetes Association (ADA) definition for at least 3 months having uncontrolled DM defined as HbA1c > 7%; stable glucose-lowering medications for at least 3 months using oral agents and/or insulin or non-insulin injectable therapy; using semaglutide for at least 6 months; and having an HbA1c readings for all time points as well as other relevant parameters like fasting blood glucose (FBG) and postprandial blood glucose (PPBG), BMI, and weight.
Patients missing any HbA1c data points or missing HbA1c during the 3 months prior to starting semaglutide, patients adding any additional diabetes medications during the retrospective period, patients with other types of DM, and participants in interventional clinical studies during the study period were excluded from the study.
The term baseline HbA1c refers to a reading obtained 3–6 months before the start of the research. The baseline HbA1c reading closest to the index date was utilized if numerous baseline HbA1c readings were available. Follow-up HbA1c measurements were those taken 6 and 12 months after starting semaglutide. Every HbA1c measurement utilized in the analysis was obtained from a laboratory test and was documented in the patient’s medical records.
The study centers also extracted data from the medical records for baseline age, gender, baseline BMI, baseline weight, baseline duration of DM, baseline insulin, baseline insulin dose if any, oral glucose-lowering medications, and baseline frequency of hypoglycemia in addition to the baseline HbA1c concentrations. Additionally, baseline measurements of systolic blood pressure (SBP), diastolic blood pressure (DBP), FBG, PPBG, lipid profile, hypertension, dyslipidemia, and medications to treat these conditions were made.
Outcomes
The primary endpoint was the laboratory HbA1c level as well as reduction at 6 and 12 months. The secondary endpoints were FBG, PPBG, weight reduction, frequency and severity of hypoglycemia in the previous 6 months, changes in lipid profile, SBP, DBP, basal insulin (BI) dose, semaglutide dose, and anti-DM drug dosage. Following the introduction of semaglutide for 6 and 12 months, all these variables were examined.
Statistical Analysis
Considering the preliminary descriptive nature of this study, a sample size calculation was not performed. Statistical analyses were performed by stratifying the study population by previous use of GLP-1RAs: GLP-1RA new users (GLP-1-naïve cohort) and patients switching from another GLP-1RA to semaglutide (GLP-1-switcher cohort).
Descriptive data were summarized as the mean and standard deviation for continuous variables or frequency and proportion for categorical variables. Baseline patient characteristics according to the study cohort were compared using the unpaired t test or the Mann–Whitney U test in case of continuous variables and the chi-square test or the Fisher exact test for categorical variables, as appropriate. Statistical significance was declared if p < 0.05.
The co-primary endpoints were the changes in HbA1c and weight after 6 months. All other changes in continuous clinical endpoints at 6 and 12 months represented secondary endpoints. In addition, the proportions of patients reaching HbA1c < 7% and weight loss > 5% were considered categorical secondary endpoints. Changes in continuous study endpoints were assessed using linear models for repeated measurements. Results are expressed as the estimated mean or difference from baseline with their standard deviations (SD). Paired t tests derived from linear mixed models for repeated measurements were applied for within-group comparisons in the naïve and switcher cohorts. Between-group comparisons were avoided because of systematic differences in the two study cohorts.
All statistical tests were carried out using a significance level of 95%. A value of P < 0.05 was considered statistically significant. SPSS software (Statistical Package for the Social Sciences, version 25.0, SSPS Inc, Chicago, IL, USA) was used for the statistical analyses. Similar methodologies to the current study have been presented elsewhere [22, 23].
Results
The analysis of this study included 1223 patients whose data were available and met the eligibility criteria from the 12 participating centers.
Baseline Characteristics
As depicted in Table 1, men accounted for 596 patients (48.7%). The mean age was 53.6 (8.2) years, the mean BMI was 38.6 (6.6) kg/m2, and the mean weight was 114.2 (19.6) kg.
About half (50.4%, 616) of the patients had hypertension. These patients received angiotensin-converting enzyme inhibitors (ACEIs) (n = 300), angiotensin receptor blockers (ARBs) (n = 305), thiazide diuretics (n = 313), and calcium channel blockers (CCBs) (n = 307). In total 588 (48.1%) of cases had dyslipidemia.
All cases were uncontrolled T2DM with an average duration of 12.3 (4.5) years. The mean (SD) HbA1c was 10.02 (1.17)%, FBG 155.8 (14.8) mg/dL, and PPBG 308.6 (28) mg/dL. About 295 (24.1%) of the cohort had an HbA1c ≤ 9%.
The entire cohort included 979 GLP1-naïve and 244 GLP1-switcher patients; 595 (48.7%) of cases received metformin for their DM, 601 (49.1%) dipeptidyl peptidase 4 inhibitors (DPP4is), 624 (51%) sulfonylureas (SUs), 606 (49.6%) BI, and 596 (48.7%) sodium-glucose cotransporter 2 inhibitors (SGLT2is). The basal insulin dose was 34.9 (14.8) units per day. The frequency of hypoglycemia events was 4.60 (1.10) in the last 3 months prior to semaglutide initiation.
GLP1-naïve and GLP1-switcher groups were comparable concerning all baseline data (p values > 0.5) except for dyslipidemia, which was more prevalent in the GLP1-switcher subgroups, as shown in Table 1.
Semaglutide was initiated in all patients. About 80% of patients had semaglutide SC injection, and 20% received semaglutide orally.
Follow-up Data
Change in Glycemic Variables
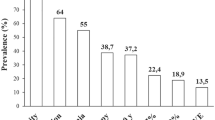
As shown in Table 2 and Fig. 1, HbA1c was reduced by an average of 3.02 (0.84) and 3.17 (0.84) at 6 and 12 months, respectively. HbA1c was 7.00 (0.70) and 6.85 (0.69) at 6 and 12 months, respectively.
A repeated measure analysis of variance (ANOVA), with Greenhouse–Geisser correction, was conducted to assess whether there were differences between HbA1c at baseline and HbA1c at 6 and 12 months. Results indicated significant differences in HbA1c (p value < 0.001). HbA1c levels at 6 and 12 months were significantly lower at 7.00 (0.70) and 6.85 (0.69) than at baseline 10.02 (1.17). About 193 (56.4%) of the 295 initially having HbA1c < 9 achieved HbA1c of 5.70 or less.
FBG was reduced by an average of 49.2 (7.3) and 57.3 (7.5) at 6 and 12 months, respectively. Also, PPBG was reduced by an average of 80.4 (6.7) and 90.4 (6.7) at 6 and 12 months, respectively.
The frequency of hypoglycemia events in the last 3 months was 2.30 (0.80) events and 0.80 (0.50) events at 6- and 12-month follow-up visits, respectively.
Change in Body Weight and BMI
The absolute reduction in body weight was an average of − 14.90 (3.06) kg and − 22.57 (6.98) kg at 6 and 12 months, respectively. The percent reduction in body weight was an average of 13.03 (1.39%) and 19.55 (3.48%) at 6 and 12 months, respectively. The percent reduction in BMI was an average of 13.07 (1.53) and 19.89 (4.07%) at 6 and 12 months, respectively.
Change in Other Cardiometabolic Parameters
Total cholesterol (TC) was reduced by an average of 40.10 (8.80) at 12 months. Low-density lipoprotein (LDL) was reduced by an average of 15.90 (2.60) at 12 months. TG was reduced by an average of 32.70 (6.50) at 12 months. High-density lipoprotein (HDL) was increased by an average of 2.50 (0.60) at 12 months. SBP and DBP were reduced by an average of 8.10 (1.20) and 5.00 (1.10) at 12 months, respectively.
Change in Anti-DM Medications
At the end-of-study SUs and DDP4is were stopped. At 6-month follow-up, the BI dose was reduced by 50.10 (10)%.
Change in Semaglutide Dose, Discontinuation of Semaglutide, and Adverse Events
At 6-month follow-up, about 428 (35%) of the population was on semaglutide 0.5 SC or its equivalent dose of the oral form (14 mg), and 795 (65%) were on 1 mg SC.
During the first 6 months, 229 cases (18.72%) out of 1223 developed gastrointestinal (GI) side effects. In 216 cases, they were mild; however, in 13 cases, they was severe to the limit that the patients stopped their semaglutide.
At the 12-month follow-up, about 244 (20.17%) of the remaining 1210 were on semaglutide 0.5 SC or its equivalent dose of the oral form, and 966 (79.83%) were on 1 mg SC.
Discussion
In this study, the authors evaluated the clinical effectiveness and safety of semaglutide in individuals with uncontrolled T2DM depending on real-world evidence from Saudi Arabia. The sample size is large enough, but the result add no new findings. However, the study aimed to reflect the real-world situation in the local setting.
The current study confirmed the efficacy and tolerability of semaglutide administered either orally or via SC injection in clinical settings with no new safety concerns. Results in both cohorts of patients who were GLP-1RA switchers from another GLP-1RA and naïve individuals demonstrate meaningful reductions in HbA1c and obesity indices and additional advantages in cardiovascular risk factors.
The study demonstrated that the benefits obtained through the SUSTAIN program [9,10,11,12,13,14] might be utilized in practical settings. In the SUSTAIN program, semaglutide 0.5 mg and 1 mg lowered HbA1c from 1.1% to 1.5% and from 1.4% to 1.8%, respectively. Weight loss from 3.5 to 4.6 kg was likewise achieved with semaglutide 0.5 mg and from 4.5 to 6.5 kg with semaglutide 1 mg. In the current study, the HbA1c decreased by 3.02 percentage points, and the weight decreased by 13.03% after 6 months.
Of course, we cannot deny that the HbA1c improvement by 3.02% in the current study was not expected with only initiation of semaglutide, even if initial HbA1c was very high as in our patients. This is not in line with prior studies concerning semaglutide. However, this result is not to be generalized as there are many factors that should be evaluated before considering it as conclusive evidence. Factors like more compliance and adherence of patients to their treatment being administered once weekly via SC injection or orally, adherence to diabetes education recommendations, or others changes in lifestyle may have contributed to this result. The result is not conclusive evidence and it warrants further prospective studies in the Saudi people before drawing a conclusion.
The study also showed that both GLP-1RA-experienced and GLP-1RA-naïve individuals had improvements in HbA1c and weight, as was expected. Although the safety profile is similar to that reported with other GLP-1RAs, semaglutide may be more effective than other GLP-1RAs, according to the available evidence [24]. Why semaglutide is more effective than the other GLP-1RAs is still a mystery. However, it is also plausible that liraglutide and semaglutide differ in this regard, and that the acyl moiety of acylated drugs like semaglutide facilitates entry into other regions of the central nervous system (CNS). Because of the chemical structure of semaglutide, it is believed that receptors in the CNS provide the weight impact by allowing access to more parts of the nervous system [25].
GLP-1RAs have also reduced dyslipidemia and lowered blood pressure, two cardiovascular (CV) risk factors [26]. Our study’s findings were consistent with existing literature, indicating improved CV risk variables. GLP-1RAs have features that can be used to treat hyperglycemia and other illnesses like CV risk factors and non-alcoholic fatty liver disease, according to an expert opinion from the Italian Diabetes Society [27].
The findings of the study are consistent with research conducted in real-world settings in many countries. A statistically significant mean decline in HbA1c of 1.03 percentage points and a weight loss of 3.9 kg were found in a study based on the retrospective analysis of a Canadian diabetic registry on 937 treatment-naïve individuals; however, there was no significant change in the self-reported incidence of hypoglycemia [28]. Semaglutide was observed to facilitate substantial weight loss (1.69 kg) and HbA1c drops (0.65% after 6 months) among individuals switching from another GLP-RA (liraglutide or dulaglutide) in another real-world trial (REALIZE-DM, N = 164) [29].
Furthermore, HbA1c reduction in naïve patients was twice as large as that in switchers (1.2% vs. 0.6%), according to data from the US Commercially Insured and Medicare Advantage Population (N = 1888); additionally, percentage point reductions in HbA1c were 2.2% among the subset of individuals with a baseline HbA1c > 9% (75 mmol/mol) [30]. Another retrospective study found that semaglutide’s persistence at 360 days was significantly higher than that of the comparator drugs (dulaglutide 56%, liraglutide 40%, and exenatide QW 35.5%), while adherence was comparable or higher [31].
Semaglutide was linked to a weight loss of 5.4 kg and a reduction in HbA1c of 1.2% in the SURE Denmark/Sweden cohort (N = 331) in Europe. After the study, 67.5% of patients had HbA1c below 7%, while 49.4% had lost at least 5% of their body weight [32]. HbA1c decreased by 0.8%, weight by 5.0 kg, and waist circumference by 4.8 cm in the SURE Switzerland cohort (N = 214) [33].
The effects of semaglutide once weekly on HbA1c and body weight in people with T2DM on a wide range of glucose-lowering treatments were also comparable to those seen in clinical studies. However, fewer people were receiving the maximum dose of semaglutide, according to real-world data from a diabetes outpatient clinic in Denmark (N = 119) [34]. In a retrospective analysis of 189 patients in Wales after 6 months, HbA1c dropped by 1.5%, and weight rose by 3 kg [35].
Semaglutide was usually well tolerated in these real-world investigations, and no new safety signals were found. Semaglutide’s good CV effects in lowering the risk of major adverse cardiovascular event (MACE) have recently been shown to have noticeable effects on HbA1c and body weight, which is exceptionally encouraging for the clinical use of the medication [18]. Semaglutide’s long-term effectiveness, safety, and effects on other endpoints (such as the fatty liver index) require further research. Additionally, orally administered semaglutide was licensed as the first oral GLP-1RA for the treatment of T2DM following the recent results of the PIONEER program [36, 37], expanding the potential of using the medication in patients who are unable to self-administer an injectable agent.
The study has both advantages and disadvantages. The fact that this is the first Saudi study to record the effects of semaglutide in the real world is its greatest strength. Another advantage is that the sample (1223 cases) is sufficient to provide more evidence. Availability of information on FBG, PPBG, lipid profile, blood pressure, side effects, and hypoglycemia is a further strength. On the other hand, the current study is not without limitations. Being retrospective in nature is one limitation. Another limitation is that no subgroup analyses were planed by age group, obesity, diabetes medications, or severity. Therefore, we cannot draw a definitive conclusion for results in those subgroups.
Conclusion
Semaglutide, administered by either SC injection or orally, was found to provide significant glycemic and weight-loss benefits in individuals with T2DM, suggesting its widespread usage in treating DM. Semaglutide is now the most potent in the GLP-1RA class, but its use requires further exploration. Patients not previously receiving insulin treatment or switching from other GLP-1RAs also benefited from the medication.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
International Diabetes Federation, IDF Diabetes Atlas, International Diabetes Federation, Brussels, Belgium, 8th edition, 2017, http://www.diabetesatlas.org.
Al-Nozha MM, Al-Maatouq MA, Al-Mazrou YY, et al. Diabetes mellitus in Saudi Arabia. Saudi Med J. 2004;25:1603–10.
Alzaid A. Diabetes; the tale of two cultures. Br J Diabetes Vasc Dis. 2012. https://doi.org/10.1177/1474651412444143.
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: tesults from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843.
World Health Organization. Diabetes 2022. https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed 8 Mar 2023.
IDF Diabetes Atlas 2021-10th edition. Saudi Arabia: Diabetes report 2010–2045. https://www.diabetesatlas.org/data/en/country/174/sa.html. Accessed 8 Mar 2023.
IDF Diabetes Atlas 2021–10th edition. Middle East and North Africa fact sheet 2019. https://diabetesatlas.org/upload/resources/material/20191218_144557_mena_factsheet_en.pdf. Accessed 8 Mar 2023.
Lau J, Bloch P, Schaffer L, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58(18):7370–80.
Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, openlabel, randomized clinical trial. Diabetes Care. 2018;41(2):258–66.
Ahren B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–54.
Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of onceweekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355–66.
Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100–9.
Lingvay I, Catarig AM, Frias JP, et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as addon to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(11):834–44.
Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–86.
Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291–301.
Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–60.
Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356–67.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Seeger JD, Nunes A, Loughlin AM. Using RWE research to extend clinical trials in diabetes: an example with implications for the future. Diabetes Obes Metab. 2020;22(Suppl 3):35–44.
Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf. 2017;26:1033–9.
US Department of Health and Human Services (HHS) Office for Civil Rights (OCR). Guidance regarding methods for de-identification of protected health information in accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. HHS.gov. 2012. https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html. Accessed 11 May 2023.
Alsifri S, Almalki A, Sam A, et al. The impact of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in patients with diabetes; observational multicenter 15-months study. Intl J Clin Med. 2022;13:391–404. https://doi.org/10.4236/ijcm.2022.138027.
Di Loreto C, Minarelli V, Nasini G, Norgiolini R, Del Sindaco P. Effectiveness in real world of once weekly semaglutide in people with type 2 diabetes: glucagon-like peptide receptor agonist naïve or switchers from other glucagon-like peptide receptor agonists: results from a retrospective observational study in umbria. Diabetes Ther. 2022;13(3):551–567. https://doi.org/10.1007/s13300-022-01218-y. Erratum in: Diabetes Ther. 2022;13(6):1251.
Holst JJ, Madsbad S. Semaglutide seems to be more effective the other GLP-1Ras. Ann Transl Med. 2017;5:505.
Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473–88.
Iorga RA, Bacalbasa N, Carsote M, et al. Metabolic and cardiovascular benefits of GLP-1 agonists, besides the hypoglycemic effect (review). Exp Ther Med. 2020;20:2396–400.
Napoli R, Avogaro A, Formoso G, et al. Beneficial effects of glucagon-like peptide 1 receptor agonists on glucose control, cardiovascular risk profile, and non-alcoholic fatty liver disease. An expert opinion of the Italian Diabetes Society. Nutr Metab Cardiovasc Dis. 2021;21:S0939–4753(21)00410–5.
Brown RE, Bech PG, Aronson R. Semaglutide once weekly in people with type 2 diabetes: real-world analysis of the Canadian LMC diabetes registry (SPARE study). Diabetes Obes Metab. 2020;22:2013–20.
Jain AB, Kanters S, Khurana R, Kissock J, Severin N, Stafford SG. Real-world effectiveness analysis of switching from liraglutide or dulaglutide to semaglutide in patients with type 2 diabetes mellitus: the retrospective REALISE-DM study. Diabetes Ther. 2021;12(2):527–36. https://doi.org/10.1007/s13300-020-00984-x.
Visaria J, Uzoigwe C, Swift C, Dang-Tan T, Paprocki Y, Willey VJ. Real-world effectiveness of once-weekly semaglutide from a US commercially insured and medicare advantage population. Clin Ther Clin Ther. 2021;43:808–21.
Uzoigwe C, Liang Y, Whitmire S, Paprocki Y. Semaglutide once-weekly persistence and adherence versus other GLP-1 RAs in patients with type 2 diabetes in a us real-world setting. Diabetes Ther. 2021;12:1475–89.
Rajamand Ekberg N, Bodholdt U, Catarig AM, et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: results from the SURE Denmark/Sweden multicentre, prospective, observational study. Prim Care Diabetes. 2021;15:871–8.
Rudofsky G, Catarig AM, Favre L, et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: results from the SURE Switzerland multicentre, prospective, observational study. Diabetes Res Clin Pract. 2021;178:108931.
Hansen KB, Svendstrup M, Lund A, Knop FK, Vilsbøll T, Vestergaard H. Once-weekly subcutaneous semaglutide treatment for persons with type 2 diabetes: real-world data from a diabetes out-patient clinic. Diabet Med Diabet Med. 2021;38: e14655.
Williams DM, Ruslan AM, Khan R, et al. Real-world clinical experience of semaglutide in secondary care diabetes: a retrospective observational study. Diabetes Ther. 2021;12:801–11.
Rodbard HW, Dougherty T, Taddei-Allen P. Efficacy of oral semaglutide: overview of the PIONEER clinical trial program and implications for managed care. Am J Manag Care. 2020;26(16 Suppl):S335–43.
Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020;22:1263–77.
Acknowledgements
The authors of the current work would like to thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Concept and Design: Abdulrahman Alsheikh, Ali Alshehri, Saad Alzahrani, Anwar A Jammah, Fahad Alqahtani, Metib Alotaibi, Raed Aldahash, Amani M Alhozali, Fahad Alsabaan, Mohammed Almehthel, Naser Aljuhani, Ali Aldabeis, Moneer Alamri, Waleed Maghawry, Naweed Alzaman, Alshaima Alshaikh, Omar M Alnozha, Emad R Issak, Saud Alsifri. Administration: Abdulrahman Alsheikh, Ali Alshehri, Saad Alzahrani, Anwar A Jammah, Fahad Alqahtani, Metib Alotaibi, Raed Aldahash, Amani M Alhozali, Fahad Alsabaan, Mohammed Almehthel, Naser Aljuhani, Ali Aldabeis, Moneer Alamri, Waleed Maghawry, Naweed Alzaman, Alshaima Alshaikh, Omar M Alnozha, Saud Alsifri. Data Collection: Abdulrahman Alsheikh, Ali Alshehri, Saad Alzahrani, Anwar A Jammah, Fahad Alqahtani, Metib Alotaibi, Raed Aldahash, Amani M Alhozali, Fahad Alsabaan, Mohammed Almehthel, Naser Aljuhani, Ali Aldabeis, Moneer Alamri, Waleed Maghawry, Naweed Alzaman, Alshaima Alshaikh, Omar M Alnozha, Saud Alsifri. Data Quality: Abdulrahman Alsheikh, Ali Alshehri, Saad Alzahrani, Anwar A Jammah, Fahad Alqahtani, Metib Alotaibi, Raed Aldahash, Amani M Alhozali, Fahad Alsabaan, Mohammed Almehthel, Naser Aljuhani, Ali Aldabeis, Moneer Alamri, Waleed Maghawry, Naweed Alzaman, Alshaima Alshaikh, Omar M Alnozha, Emad R Issak, Saud Alsifri. Statistical Analysis: Emad R Issak. Drafting the Manuscript: Abdulrahman Alsheikh, Ali Alshehri, Saad Alzahrani, Anwar A Jammah, Fahad Alqahtani, Metib Alotaibi, Raed Aldahash, Amani M Alhozali, Fahad Alsabaan, Mohammed Almehthel, Naser Aljuhani, Ali Aldabeis, Moneer Alamri, Waleed Maghawry, Naweed Alzaman, Alshaima Alshaikh, Omar M Alnozha, Emad R Issak, Saud Alsifri. Revising the Manuscript: Abdulrahman Alsheikh, Ali Alshehri, Saad Alzahrani, Anwar A Jammah, Fahad Alqahtani, Metib Alotaibi, Raed Aldahash, Amani M Alhozali, Fahad Alsabaan, Mohammed Almehthel, Naser Aljuhani, Ali Aldabeis, Moneer Alamri, Waleed Maghawry, Naweed Alzaman, Alshaima Alshaikh, Omar M Alnozha, Emad R Issak, Saud Alsifri.
Corresponding author
Ethics declarations
Conflict of Interest
Abdulrahman Alsheikh, Ali Alshehri, Saad Alzahrani, Anwar A Jammah, Fahad Alqahtani, Meteb Alotaibi, Raed Aldahash, Amani M Alhozali, Fahad Alsabaan, Mohammed Almehthel, Naser Aljuhani, Ali Aldabeis, Moneer Alamri, Waleed Maghawry, Naweed Alzaman, Alshaima Alshaikh, Omar M Alnozha, Emad R Issak and Saud Alsifri have nothing to disclose.
Ethical Approval
The study conformed to the principles of the 2011 Declaration of Helsinki and the Good Pharmacoepidemiology Practices (GPP) guidelines. An accredited centralized institutional review board (IRB) approved the study, and informed consent was waived by the IRB as the study was retrospective. The central IRB approval covered the necessary ethics approval for all study sites. Informed consent was not required as the study is retrospective chart review study and all data is HIPAA de-identified.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alsheikh, A., Alshehri, A., Alzahrani, S. et al. Evaluating the Clinical Effectiveness and Safety of Semaglutide in Individuals with Uncontrolled Type 2 Diabetes. Real-World Evidence from Saudi Arabia: The Observational, Multicenter, 15-Month EVOLUTION Study. Diabetes Ther 15, 473–485 (2024). https://doi.org/10.1007/s13300-023-01516-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01516-z