Abstract
Introduction
The psychological burden of type 1 diabetes mellitus (T1DM) is considerable. The condition affects the daily lives of adults living with T1DM (ALWT1DM) in many ways. International guidelines highlight the importance of providing psychological support to ALWT1DM to improve health outcomes and well-being.
Methods
We conducted a systematic literature review of randomised controlled trials (RCTs) to identify the evidence on the impact of psychological interventions on glycaemic control and psychological outcomes in ALWT1DM. Literature searches of Medline, Embase, Cochrane Central Register of Controlled Trials, PsycInfo, and the grey literature were performed to identify relevant RCTs, published in English, from 2001 onward. Fourteen RCTs of ten psychological interventions in ALWT1DM were eligible and included in the qualitative synthesis. The studies varied considerably in terms of duration, target population, endpoints, and efficacy.
Results
Overall, psychological interventions did not perform significantly better than control treatments in improving glycaemic control, although selected patient groups did report benefits from some psychological intervention types, such as cognitive behavioural therapy. Although most of the psychological interventions produced small, nonsignificant improvements in self-reported patient functioning, some treatments were effective in reducing diabetes distress and improving mental health, even if no impact on glycaemic control was observed.
Discussion
Current guidelines for the treatment of T1DM recommend access to psychological services; however, there is a paucity of high-quality evidence from clinical trials on the effectiveness or preferred structure of psychological support. There is a clear need for more rigorous, large-scale, international research to address the efficacy of psychological interventions in ALWT1DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Although international guidelines emphasize the significance of providing psychological support to adults living with type 1 diabetes mellitus (ALWT1DM) to improve their health outcomes and overall well-being, previous systematic reviews primarily focused on assessing the impact of psychological treatments on glycaemic control, without considering psychological outcomes. |
This systematic review aims to contribute to the existing evidence by providing a comprehensive summary of the efficacy of psychological interventions in ALWT1DM, evaluating the impact of these interventions on both glycaemic control and psychological outcomes. |
What was learned from the study? |
This study suggests that psychological interventions in ALWT1DM did not significantly improve glycaemic control overall, but certain patient groups benefited. |
More large-scale and rigorous research is needed to determine the efficacy of psychological support in ALWT1DM. |
Introduction
Type 1 diabetes mellitus (T1DM) is characterised by deficient insulin production, which requires daily administration of insulin to avoid persistent and progressive hyperglycaemia, diabetic ketoacidosis, and death. Its prevalence and incidence are increasing worldwide, although it remains more common in certain populations and geographies. It has been estimated that T1DM represents approximately 5–10% of the total prevalence of diabetes, corresponding to 21–42 million people worldwide [1].
The psychological burden of T1DM is considerable for the patients and family carers [2]. The condition affects lifestyle and daily living in many ways, potentially impacting the enjoyment of normal activities and social life. A high focus has been placed on people with diabetes engaging in self-management and becoming effective caretakers of their own condition. Successful self-management of T1DM involves frequent blood glucose monitoring, carbohydrate counting, and calculations of insulin dose to achieve optimal glycaemic control and avoid diabetes-related complications and the complications of treatment, particularly hypoglycaemia [3]. Learning that T1DM is a chronic, currently incurable disease can have a major emotional toll on individuals [4]. Because T1DM is a lifelong condition, self-discipline must be maintained every hour of every day for a lifetime. Any obstacles in keeping the self-management rigour can be experienced as a loss of control that can lead to reduced quality of life [5].
Because T1DM self-management remains highly complex and, for many, psychologically demanding [6], international guidelines suggest that adults living with T1DM (ALWT1DM) should receive screening and psychological support in order to treat common psychological problems and relieve the daily stress of diabetes self-management [3]. Previous systematic literature reviews of psychological interventions in ALWT1DM were focused mainly on the assessment of the psychological treatments’ impact on glycaemic control and did not consider psychological outcomes [7].
Therefore, the main goal of this study was to systematically review and summarise the evidence from randomised controlled trials (RCTs) on the impact of psychological interventions on glycaemic control and psychological outcomes in ALWT1DM.
Methods
We conducted a systematic literature review (SLR) and qualitative synthesis of RCTs of psychological interventions in ALWT1DM. We followed the standards set out in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [8] and the Cochrane Handbook for Systematic Reviews of Interventions [9]. We employed the Cochrane risk-of-bias tool to assess study quality [10].
Data Sources and Searches
Searches were run via Ovid on 5 January 2022, in Medline, Embase, Cochrane Central Register of Controlled Trials, and PsycInfo. The terms encompassing diabetes mellitus, psychological interventions, and RCTs were used to search Embase and adjusted for the other databases. The search strategy is detailed in Table S1 in the electronic supplementary material.
Reference lists of relevant previously published SLRs were manually searched for additional articles. The conference proceedings from key diabetes conferences (American Diabetes Association [ADA], European Association for the Study of Diabetes [EASD], Advanced Technologies & Treatments for Diabetes [ATTD], American Psychological Association [APA], PsychoSocial Aspects of Diabetes [PSAD], and Diabetes UK) were searched to identify recent, relevant research from the past 2 years that may not have been published in peer-reviewed journals.
Study Selection
We included RCTs of psychological interventions for adults (≥ 18 years) with T1DM, published in English, between 2001 and 2022. Studies combining type 1 and type 2 diabetes or children (< 18 years) with adults were excluded unless results were stratified by type of diabetes or by age group, respectively. Studies were not excluded on the basis of setting, delivery, or duration of the intervention. Detailed inclusion and exclusion criteria are provided in Table S2 in the electronic supplementary material. Study selection was performed by two independent reviewers; when necessary, a third reviewer resolved any discrepancies.
Data Extraction and Quality Assessment
Extracted data included publication characteristics, study details, patient characteristics and results. The risk of bias of the included studies was assessed using the RoB 2.0 tool [10]. Bias was assessed as “low risk”, “high risk”, and “some concerns”; the last category indicated either a lack of information or uncertainty over the potential for bias. Data were extracted and assessed for risk of bias from each included publication by a single investigator, and validation was performed by a second reviewer.
Results
Study Selection
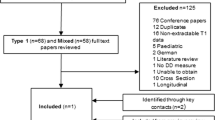
The literature search identified 1554 articles from the electronic databases, 18 of which met the inclusion criteria. One additional publication was obtained though bibliography screening. A total of 19 publications reporting the results of 14 unique studies were included in the narrative synthesis. The study attrition is presented in Fig. 1.
Study Overview
Study Design
The design of the studies identified was comparable across most of the domains. The evidence comprised mostly open-label (n = 8) [11,12,13,14,15,16,17,18], multicentre trials (n = 9) [11, 13, 16,17,18,19,20,21,22] with study sites in Western Europe and North America. The included studies were heterogeneous in terms of size and follow-up duration. The sample sizes of patients randomised in eligible studies varied widely, ranging from 46 patients [12] to 345 patients [13]. The duration of follow-up ranged from 2 months [23] to 18 months [18]; almost half of the studies investigating a long-term effect of psychological interventions had at least a 12-month follow-up. Of the included trials, only four were relatively recent [13, 14, 18, 20], enrolling patients in the past 10 years.
Most of the trials included in the SLR were rated as having a high risk of bias. Two studies were assessed as having a low risk of bias [23, 24], and one trial [13] had some concerns. One trial with results published as a conference abstract could not be assessed for risk of bias because of its limited information [15]. The factor that contributed the most to a high risk of bias included a high risk around the measurement of outcome resulting from lack of adequate blinding, which is typical in trials of psychological interventions.
Detailed risk-of-bias assessment is provided in Table S4 in the electronic supplementary material.
Treatment Characteristics
Fourteen eligible RCTs assessed ten various psychological interventions. Most of the trials investigated the efficacy of cognitive behavioural therapy (CBT) (n = 4) [11, 20,21,22], guided self-determination (GSD) (n = 3) [14, 17, 18], and blood glucose awareness training (BGAT) (n = 2) [16, 22]. The most common control groups included usual care (n = 5) [11, 14,15,16, 19] and waiting list (n = 4) [12, 17, 18, 20]. Most psychological interventions were administered by diabetes nurses (n = 7) [11, 14, 16,17,18,19, 22], followed by clinical psychologists (n = 6) [11,12,13, 20,21,22]. CBT-based interventions tended to involve psychologists [11, 20,21,22], whereas GSD-based treatments tended to be fully administered by nurse specialists [14, 17, 18]. The majority of interventions were delivered face to face (n = 12) [11, 12, 14,15,16,17,18,19,20,21,22,23].
Detailed study characteristics are provided in Table 1.
Patient Characteristics
The populations enrolled in the identified trials differed broadly across demographic and clinical characteristics. Twelve trials reported patients’ mean age [11,12,13,14,15,16,17,18, 20,21,22, 24], ranging across treatment arms from 20.4 years [24] to 52.7 years [16]. The mean baseline haemoglobin A1c (HbA1c; n = 14) [11,12,13,14,15,16,17,18,19,20,21,22,23,24] levels varied across treatment arms from 7.7% [16] to 10.4% [23]. Most trials (n = 11) reported diabetes duration [11,12,13,14, 16, 18,19,20,21,22, 24], and mean values ranged widely across treatment arms from 8.9 years [24] to 27.5 years [16]. The data on proportion of patients with diabetes complications at baseline were underreported (n = 5) [12, 14, 16, 18, 22]. Overall, the proportion of patients with any complications ranged from 22.9% [14] to as high as 56% [16]. One trial [12] exclusively enrolled patients who had at least one diabetes complication. Trials were broadly similar in terms of patients’ body mass index (BMI) (n = 7) [11, 12, 14, 16, 18,19,20], which ranged from 24.5 kg/m2 [18] to 26.2 kg/m2 [20]. The sex distribution was comparable across trials.
Detailed patient characteristics are provided in Table S3 in the electronic supplementary material.
Study Results
HbA1c
All of the RCTs included in the SLR (n = 14) reported the impact of psychological interventions on the levels of HbA1c. The HbA1c levels dropped over time in most of the psychological intervention arms but less so in the control arms. Overall, psychological treatments were not more effective than comparator treatments in improving glycaemic control. Limited evidence suggested that relaxation training [23] could lead to a significant improvement in glycaemic control at a short-term follow-up of 2 months, whereas CBT [11, 19] significantly decreased HbA1c levels at 12 months compared with the control group.
Two trials addressed the head-to-head relative efficacy of psychological interventions, including short-term (3 months) comparison of BGAT with CBT [22] and longer-term (12 months) comparison of motivational enhancement therapy (MET) with MET + CBT [22]. Although no significant differences were observed between BGAT and CBT, the combination of CBT and MET performed better than MET alone in improving the glycaemic control compared with the control group.
Detailed results are shown in Fig. 2 and Table S5 in the electronic supplementary material.
Quality of Life
Four studies investigated the overall quality of life in ALWT1DM, using generic measures such as the EuroQol EQ-5D (n = 1, telehealth vs. telehealth + virtual group appointment [VGA] [24]), the 12-item Well-Being Questionnaire (W-BQ12; n = 1, CBT vs usual care [11]), Indicators of the Rehabilitation Status (n = 1, psychotherapy vs. waiting list [12]), and diabetes-specific questionnaires such as the Diabetes Quality of Life questionnaire (n = 1, MET, MET + CBT vs. usual care [19]). Study results indicated that only CBT significantly improved well-being of ALWT1DM, as measured with the W-BQ12 questionnaire at 12-month follow-up compared with usual care [11].
Detailed results are shown in Table S6 in the electronic supplementary material.
Mental Health
Ten studies investigated the mental health outcomes in ALWT1DM, using instruments such as the Center for Epidemiologic Studies Depression Scale (CES-D; n = 2, cognitive behavioural group training [CBGT] vs. BGAT [24], telehealth + VGA vs. VGA [22]), the WHO-5 Well-Being Index (WHO-5; n = 2, GSD vs. usual care [14], flexible GSD vs. usual care [18]), and others, including the 17-item Hamilton Depression Rating Scale (n = 1, CBT vs. sertraline [21]), the Hospital Anxiety and Depression Scale (HADS; n = 1, BGAT vs. usual care [16]), the Patient Health Questionnaire-9 (n = 1, MET, MET + CBT vs. usual care [19]), the Perceived Stress Scale (PSS; n = 1, CBT vs. usual care [11]), the Symptom Checklist-90-Revised (n = 1, psychotherapy vs. waiting list [12]), and self-reported depression rates (n = 1, CBT vs. sertraline [21]).
Although CES-D depressive symptoms were reduced over time in the CBT, BGAT [25], and telehealth + VGA treatment arms [24], no significant benefit was observed compared with the control group. The results for the WHO-5 were mixed; increased emotional well-being over time was reported for the comparison of flexible GSD with waiting list at the 18-month follow-up [18], and deterioration was observed in the study comparing GSD with usual care at the 9-months follow-up [14]. No significant benefit was observed when compared with the control group. Of the remaining measures, statistically significant improvements were found in the total PSS and HADS for the comparison of CBT [11] and BGAT [16], respectively, with the usual care.
Detailed results are shown in Table S7 in the electronic supplementary material.
Diabetes Distress
Eight studies investigated the diabetes distress outcomes in ALWT1DM using instruments such as the 20-item Problem Areas in Diabetes (PAID-20; n = 5) [11, 14, 17, 18, 22]), the Diabetes Distress Scale (DDS; n = 3, GSD vs. usual care [14], emotion-focused intervention vs. education [13], telehealth + VGA vs. VGA [22]), the Hypoglycemia Fear Survey (HFS; n = 1, CBT vs. usual care [11]), and five-item PAID (PAID-5; n = 1, BGAT vs. usual care [16]).
Although a greater numerical reduction of PAID-20 total scores compared with the control group was observed in all of the studies reporting PAID-20 interventions, only GSD [14, 18] significantly reduced the diabetes distress compared with control intervention, whereas CBT [22] did not lead to any significant changes. No significant difference in diabetes distress on the PAID-5 score was found [16].
The total DDS scores decreased over time in most of the study arms. GSD [14] and telehealth + VGA [24] significantly improved total DSS compared with control. Compared with usual care, CBT treatment [11] provided no significant benefit related to diabetes distress [11] measures using HFS.
Detailed results are shown in Table S8 in the electronic supplementary material.
Other Outcomes
Six studies investigated self-efficacy in ALWT1DM using instruments such as the Perceived Competence in Diabetes Scale-5 (PCDS-5 (n = 2, GSD vs. usual care [14], GSD vs. waiting list [18]), PCDS-3 (n = 1, GSD vs waiting list [17]), Confidence in Diabetes Self-Care (CIDS; n = 2, CBT vs. waiting list [20], CBT vs. BGAT [22]), and Self-Efficacy for Diabetes Scale (n = 1, telehealth + VGA vs. VGA [22]). A significant improvement was observed only in total CIDS at 5-month follow-up compared with baseline in patients receiving CBT [24].
Other outcomes of interest were sparsely reported in the literature. Two RCTs reported the impact of psychological interventions on episodes of hypoglycaemia (BGAT vs. usual care [16], MET, MET + CBT vs. usual care [19]). The mean number of severe hypoglycaemia episodes dropped over time in psychological intervention arms; however, the differences observed were not significant. Limited evidence was found for patient-reported outcomes (PROs) such as self-monitoring of blood glucose (SMBG; n = 3, GSD vs. usual care [14], CBT vs. BGAT [22], GSD vs. waiting list [17]); patient autonomy support, as measured by the Health Care Climate Questionnaire (n = 3, GSD vs. usual care [14], GSD vs. waiting list [17], flexible GSD vs. waiting list [18]); self-esteem, as measured by the Rosenberg Self-Esteem Scale (n = 2, GSD vs. usual care [14], flexible GSD vs. waiting list [18]); adaptive behaviours, as measured by the Diabetes Strengths and Resilience Measure for Adolescents (n = 1, telehealth + VGA vs. VGA [22]) and Gold score (n = 1, BGAT vs. usual care [16]). Significant improvements in SMBG frequency [14], patient autonomy support [17, 18], and self-esteem [14] were observed for GSD compared with control group. BGAT resulted in significant reductions in impaired hypoglycaemia awareness, as defined by Gold score [16].
Healthcare resource utilisation (HCRU) was measured in a single trial, comparing MET and MET + CBT with usual care [19]. The hospitalisation and outpatient services utilisation rates were stable over time and broadly similar across study arms.
Discussion
Summary of Results
In total, we identified 14 unique RCTs assessing psychological interventions for improving management of T1DM in adults. Overall, psychological interventions were not significantly better than control interventions in improving glycaemic control. However, some specific interventions, such as relaxation training [23] and CBT [11, 19], were associated with significant reduction in HbA1c levels compared with no psychological treatment for ALWT1DM. These findings are consistent with a previously published SLR [7]. Patients with poor baseline glycaemic control [11, 19] and younger patients [11, 19] tended to benefit more than other populations. The included studies assessed the impact of psychological interventions on various areas of self-reported patient functioning. Most of the psychological interventions produced small, nonsignificant improvements compared with the control group. However, some evidence suggested that patients may significantly benefit from CBT [11, 16], GSD [14, 17, 18], BGAT [16], and VGA [24], mainly in terms of improved mental health and reduced diabetes distress, even if no improvements in glycaemic control were observed.
Strengths and Weaknesses
The strengths of this study are the comprehensive approach towards finding all relevant literature and critical appraisal of the quality of evidence to obtain the robust and valid evidence for psychological interventions in the treatment of ALWT1DM.
Nevertheless, it has limitations that affect the interpretability of the results and conclusions. First, heterogeneity was identified in the baseline patient characteristics across the different included trials; thus, the naive comparison of the numeric results across the studies should be interpreted with caution.
Most of the trials employed an open-label design. The lack of blinding in many of the included RCTs can be explained by the fact that any attempts of blinding psychological interventions may encounter some inherent obstacles related to the nature of the interventions themselves. Recent research supports the validity of PRO results from open-label trials [26]. Thus, a lack of adequate blinding should not impact the interpretation of the results from the included trials.
Several different PRO measures were used to assess the impact of psychological interventions on ALWT1DM; most of the PRO instruments were implemented in a single trial, limiting opportunities to compare the results across trials [14, 18].
The most common control group in included trials was usual care [11, 14,15,16, 19]. Nonetheless, any comparisons must be interpreted with caution, because the definitions of ‘usual care’ and the level of care provided to ALWT1DM within this control group were heterogeneous.
Implications for Research
Most of the studies in which psychological interventions demonstrated a significant clinical or psychological benefit enrolled patients with poor baseline glycaemic control [11, 19] and younger patients [11, 19], which suggests that these groups of ALWT1DM may benefit the most from psychological interventions.
In some trials, high dropout rates were observed [13, 14, 20,21,22, 24], and these trials tended to have longer treatment duration and to report nonsignificant outcomes more often than did other studies. Our findings are congruent with previously published research suggesting that shorter duration psychological intervention is characterised by better compliance and greater patient benefit in terms of the glycaemic control [27]. This elevated attrition rate highlights the need to develop a psychological intervention that will engage individuals and will be easily accepted by ALWT1DM for the longer term. To have a greater chance of being successful, psychological treatment of T1DM should be more individualised, taking into account patient profile and preferences.
Compared with HbA1c, time in range is a more dynamic glycaemic control measure, allowing highs, lows, and in-range values to be captured and offering a nuanced, cause-and-effect understanding of diabetes [28, 29]. We did not identify studies that assessed the impact of psychological interventions on time in range. Similarly, this SLR found no studies investigating diabetes-related complications, which are considered to be a key driver of the economic burden in patients with diabetes [30, 31]. This SLR identified one study that focused on HCRU [19]. RCTs examining HCRU outcomes in ALWT1DM are needed to provide specific data.
In all of the included trials, the impact of the psychological interventions on the glycaemic control in patients living with T1DM was the primary outcome of interest. However, in many instances, the primary goal of psychological treatment in T1DM is not necessarily to reduce HbA1c level but rather to improve patient well-being. Future studies should focus more on exploring the psychological outcomes as primary study endpoints.
Another evidence gap is the paucity of recent research, given that only four [13, 14, 18, 32] of the included trials were relatively recent, having enrolled patients in the last 10 years. Nonetheless, it should be borne in mind that the concept of psychological interventions is distinct from that of standard pharmacological interventions, and some additional considerations should be taken into account when designing clinical trials [33]. The process of providing data on the efficacy of psychological interventions is more complex; thus, naturally, less evidence is published in this area. The applicability of the findings from the older studies to current clinical practice may be questioned, so more research is needed to justify and optimise psychological support of ALWT1DM.
The findings of this SLR indicate that both CBT- and GSD-based interventions may be effective for improving glycaemic control and PROs in people living with T1DM; the former tended to involve psychologists and the latter, diabetes nurses. This suggests that the psychological interventions provided by nonpsychologists may produce effects equal to those delivered by qualified psychological therapists, which is a promising finding in light of settings with limited access to psychologists, especially in the COVID-19 pandemic era [34]. These findings need to be interpreted with caution, given the limitations of naive comparisons, especially lack of control for confounding factors. More research is needed to test this hypothesis, preferably in an RCT setting.
The included studies have numerous design-related limitations. First, all of the studies we identified were single-country trials, conducted mostly in the USA and northern and western European countries, meaning that the results may not be generalisable to other regions. Second, the primary objective of the reviewed research was to assess the effects of psychological interventions on glycaemic control, and the psychological outcomes were secondary study endpoints for which the trials may not have been adequately powered. Finally, the studies we included generally did not undertake any measures to adjust for the baseline imbalances across study arms, which is especially important in the setting of association between a baseline covariate and the outcome measure (e.g. HbA1c level). There is an unmet need for high-quality, large-scale, international research to address the impact of psychological interventions on ALWT1DM.
Conclusions
This SLR identified 14 RCTs investigating the efficacy of psychological interventions in the treatment of ALWT1DM. The studies reviewed varied considerably in terms of duration, population, endpoints, and efficacy, limiting opportunities to synthesise the results. Given the numerous study design limitations observed in the included studies, any findings need to be interpreted with caution. Overall, psychological interventions did not perform significantly better than control treatments in improving glycaemic control. However, several studies indicated that, for some patient groups and in response to certain psychologic interventions, ALWT1DM may benefit in terms of glycaemic control and PROs. Although guidelines for the treatment of T1DM recommend access to psychological services, there is a paucity of recent clinical trials assessing the efficacy of psychological interventions on non-HbA1c-based endpoints in ALWT1DM. This highlights the need for well-designed, randomised clinical trials on psychological interventions that will address the evidence gaps and will guide the most suitable patient and intervention profile for psychological support.
Data Availability
Data sharing is not applicable because no datasets were generated and/or analyzed for this study. All data relevant to the study are included in the article or uploaded as supplemental material.
References
Green A, Hede SM, Patterson CC, et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia. 2021;64(12):2741–50. https://doi.org/10.1007/s00125-021-05571-8.
van Duinkerken E, Snoek FJ, de Wit M. The cognitive and psychological effects of living with type 1 diabetes: a narrative review. Diabet Med. 2020;37(4):555–63. https://doi.org/10.1111/dme.14216.
Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126–40. https://doi.org/10.2337/dc16-2053.
Cho MK, Kim MY. What affects quality of life for people with type 1 diabetes? A cross-sectional observational study. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18147623.
Benkel I, Arnby M, Molander U. Living with a chronic disease: a quantitative study of the views of patients with a chronic disease on the change in their life situation. SAGE Open Med. 2020. https://doi.org/10.1177/2050312120910350.
Reddy M, Rilstone S, Cooper P, Oliver NS. Type 1 diabetes in adults: supporting self management. BMJ. 2016;352: i998. https://doi.org/10.1136/bmj.i998.
Winkley K, Upsher R, Stahl D, et al. Systematic review and meta-analysis of randomized controlled trials of psychological interventions to improve glycaemic control in children and adults with type 1 diabetes. Diabet Med. 2020;37(5):735–46. https://doi.org/10.1111/dme.14264.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). 2021. https://training.cochrane.org/handbook.
Cochrane Library. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2.0). 2019. https://methods.cochrane.org/risk-bias-20-tool.
Amsberg S, Anderbro T, Wredling R, et al. A cognitive behavior therapy-based intervention among poorly controlled adult type 1 diabetes patients—a randomized controlled trial. Patient Educ Couns. 2009;77(1):72–80. https://doi.org/10.1016/j.pec.2009.01.015.
Didjurgeit U, Kruse J, Schmitz N, Stuckenschneider P, Sawicki PT. A time-limited, problem-orientated psychotherapeutic intervention in type 1 diabetic patients with complications: a randomized controlled trial. Diabet Med. 2002;19(10):814–21. https://doi.org/10.1046/j.1464-5491.2002.00811.x.
Fisher L, Hessler D, Polonsky WH, et al. T1-REDEEM: a randomized controlled trial to reduce diabetes distress among adults with type 1 diabetes. Diabetes Care. 2018;41(9):1862–9. https://doi.org/10.2337/dc18-0391.
Mohn J, Graue M, Assmus J, et al. The effect of guided self-determination on self-management in persons with type 1 diabetes mellitus and HbA1c ≥64 mmol/mol: a group-based randomised controlled trial. BMJ Open. 2017;7(6):e013295. https://doi.org/10.1136/bmjopen-2016-013295.
Morillas Jimenez V, Ruiz De Adana M, et al. Randomized trial to evaluate the addition of a mobile app (social diabetes) in adults with type 1 diabetes. Diabetes Technol Ther. 2020;22(Suppl 1):A-163. https://doi.org/10.1089/dia.2020.2525.abstracts.
Rondags SM, de Wit M, Twisk JW, Snoek FJ. Effectiveness of Hypoaware, a brief partly web-based psychoeducational intervention for adults with type 1 and insulin-treated type 2 diabetes and problematic hypoglycemia: a cluster randomized controlled trial. Diabetes Care. 2016;39(12):2190–6. https://doi.org/10.2337/dc16-1614.
Zoffmann V, Lauritzen T. Guided self-determination improves life skills with type 1 diabetes and A1C in randomized controlled trial. Patient Educ Couns. 2006;64(1–3):78–86. https://doi.org/10.1016/j.pec.2005.11.017.
Zoffmann V, Vistisen D, Due-Christensen M. Flexible guided self-determination intervention for younger adults with poorly controlled type 1 diabetes, decreased HbA1c and psychosocial distress in women but not in men: a real-life RCT. Diabet Med. 2015;32(9):1239–46. https://doi.org/10.1111/dme.12698.
Ismail K, Thomas SM, Maissi E, et al. Motivational enhancement therapy with and without cognitive behavior therapy to treat type 1 diabetes: a randomized trial. Ann Intern Med. 2008;149(10):708–19.
Menting J, Tack CJ, van Bon AC, et al. Web-based cognitive behavioural therapy blended with face-to-face sessions for chronic fatigue in type 1 diabetes: a multicentre randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(6):448–56. https://doi.org/10.1016/S2213-8587(17)30098-0.
Petrak F, Herpertz S, Albus C, et al. Cognitive behavioral therapy versus sertraline in patients with depression and poorly controlled diabetes: the Diabetes and Depression (DAD) study: a randomized controlled multicenter trial. Diabetes Care. 2015;38(5):767–75. https://doi.org/10.2337/dc14-1599.
van der Ven NC, Hogenelst MH, Tromp-Wever AM, et al. Short-term effects of cognitive behavioural group training (CBGT) in adult type 1 diabetes patients in prolonged poor glycaemic control. A randomized controlled trial. Diabet Med. 2005;22(11):1619–23. https://doi.org/10.1111/j.1464-5491.2005.01691.x.
Paschali AA, Peppou L, Benroubi M. Relaxation training significantly reduced blood glucose levels in patients with type 1 diabetes mellitus. Hormones. 2020;19(2):215–22. https://doi.org/10.1007/s42000-020-00187-w.
Bisno DI, Reid MW, Fogel JL, Pyatak EA, Majidi S, Raymond JK. Virtual group appointments reduce distress and improve care management in young adults with type 1 diabetes. J Diabetes Sci Technol. 2021;16(6):1419–27. https://doi.org/10.1177/19322968211035768.
Snoek FJ, Van Der Ven NCW, Twisk JWR, et al. Cognitive behavioural therapy (CBT) compared with blood glucose awareness training (BGAT) in poorly controlled type 1 diabetic patients: long-term effects on HbA1c moderated by depression. A randomized controlled trial. Diabet Med. 2008;25(11):1337–42. https://doi.org/10.1111/j.1464-5491.2008.02595.x.
Efficace F, Cella D, Aaronson NK, et al. Impact of blinding on patient-reported outcome differences between treatment arms in cancer randomized controlled trials. J Natl Cancer Inst. 2022;114(3):471–4. https://doi.org/10.1093/jnci/djab177.
Yang X, Li Z, Sun J. Effects of cognitive behavioral therapy-based intervention on improving glycaemic, psychological, and physiological outcomes in adult patients with diabetes mellitus: a meta-analysis of randomized controlled trials. Front Psychiatry. 2020;11:711. https://doi.org/10.3389/fpsyt.2020.00711.
Vigersky RA, McMahon C. The relationship of hemoglobin A1c to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81–5. https://doi.org/10.1089/dia.2018.0310.
Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–9. https://doi.org/10.2337/dc17-0636.
Hu H, Sawhney M, Shi L, et al. A systematic review of the direct economic burden of type 2 diabetes in china. Diabetes Ther. 2015;6(1):7–16. https://doi.org/10.1007/s13300-015-0096-0.
Wang W, Fu CW, Pan CY, et al. How do type 2 diabetes mellitus-related chronic complications impact direct medical cost in four major cities of urban China? Value Health. 2009;12(6):923–9. https://doi.org/10.1111/j.1524-4733.2009.00561.x.
Menting J, Nikolaus S, Wiborg JF, et al. A web-based cognitive behaviour therapy for chronic fatigue in type 1 diabetes (Dia-Fit): study protocol for a randomised controlled trial. Trials. 2015;16:262. https://doi.org/10.1186/s13063-015-0764-4.
Guidi J, Brakemeier E-L, Bockting CL, et al. Methodological recommendations for trials of psychological interventions. Psychother Psychosom. 2018;87(5):276–84.
Alleaume C, Verger P, Peretti-Watel P. Psychological support in general population during the COVID-19 lockdown in France: needs and access. PLoS One. 2021;16(5):e0251707. https://doi.org/10.1371/journal.pone.0251707.
Medical Writing and Editorial Assistance
The authors thank Rodney Atkins, ELS, an employee of Evidera, for his writing and editing assistance, and Catherine Rolland, an employee of Evidera, for her support with review of the final manuscript.
Funding
This study (design and conduct, data collection, management, analysis and interpretation of the data, review, approval of the manuscript, and journal Rapid Service Fee) was funded by Sanofi.
Author information
Authors and Affiliations
Contributions
Onyinye Diribe, Karen Palmer, Adee Kennedy, and Cecile Dessapt-Baradez were involved in conception, design, interpretation, and critical revision of the manuscript. Mike Baxter was involved in the conception, design, data acquisition, analysis, interpretation, and critical revision of the manuscript. Katarzyna Borkowska was responsible for data acquisition, analysis, interpretation, and drafting and critical revision of the manuscript. Marissa Betts was involved in data analysis interpretation, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Adee Kennedy, Cecile Dessapt-Baradez, Karen Palmer, and Onyinye Diribe are employees of Sanofi and may hold share/stock in Sanofi. Marissa Betts and Katarzyna Borkowska are employed by Evidera, which provides consulting and other research services to pharmaceutical, medical device, and related organizations. In their salaried positions, they work with a variety of companies and organizations and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Mike Baxter is CEO/MD of Endotech, a company providing private medical services to patients with diabetes and endocrine conditions. He is deputy Chairman of Frimley Health and is a non-executive member of the trusts board. He is also an honorary professor of medicine at the University of Swansea. In the past, he has been involved in consultative roles for a number of commercial organisations but is not currently receiving any payment from any agencies and did not receive any payment for his involvement in this project/manuscript.
Ethical Approval
Ethics committee approval was not required for this systematic review because it involved the synthesis and analysis of existing data from previously published studies. A systematic review does not involve direct contact with human subjects or the collection of new data. Therefore, obtaining ethics committee approval was deemed unnecessary for this study. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Diribe, O., Palmer, K., Kennedy, A. et al. A Systematic Literature Review of Psychological Interventions for Adults with Type 1 Diabetes. Diabetes Ther 15, 367–380 (2024). https://doi.org/10.1007/s13300-023-01513-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01513-2