Abstract
Introduction
The aim of the study was to evaluate the efficacy and safety of fixed-dose combination (FDC) of dapagliflozin (10 mg) and linagliptin (5 mg) in comparison to linagliptin 5 mg (Trajenta) in patients with insufficiently controlled type 2 diabetes mellitus (T2DM) on metformin monotherapy.
Methods
The double-blind, randomized, multicentric, parallel-group phase III trial screened 287 adult patients with T2DM (age 18–65 years) from 16 sites across India. The recruited subjects were undergoing metformin monotherapy ≥ 1000 mg/day for at least 28 days. Patients with HbA1c of 7.5–10.5% (58–91 mmol/l) (n = 232) after 2 weeks of run-in period with linagliptin monotherapy and placebo dapagliflozin/linagliptin on metformin monotherapy were randomized (1:1) in parallel to once daily dapagliflozin/linagliptin 10/5 mg or linagliptin 5 mg for 16 weeks. Patients were stratified on the basis of HbA1c (≤ 9.0% and > 9.0%; ≤ 75 mmol/l and > 75 mmol/l)). A total of 225 subjects completed 16 weeks of treatment, 115 patients in the test group and 110 patients in the reference group.
Results
Dapagliflozin/linagliptin (p = 0.0003) exhibited a greater change in HbA1c from baseline than linagliptin (p < 0.0001) in 16 weeks (mean reduction, − 1.28% vs − 0.83%). Test group showed a significant decrease in fasting plasma glucose (FPG), postprandial plasma glucose (PPG) and body weight compared to the reference group. The FDC was well tolerated with adverse events being more frequent in the reference group. No serious adverse events (SAEs) were reported in the study.
Conclusion
Dapagliflozin/linagliptin combination is a novel dipeptidyl peptidase 4 (DPP4)/sodium-glucose co-transporter 2 (SGLT2) inhibitor FDC approved in India for patients with T2DM. Potential limitations of this study are a small dose of dapagliflozin (10 mg) in the FDC, a short study duration (30 weeks) and a high minimum threshold for HbA1c (≤ 7.5%; ≤ 53 mmol/l). Results indicate the FDC to be a superior therapeutic option over linagliptin for patients with T2DM on metformin monotherapy.
Trial Registration
CTRI/2022/08/044563; 01/08/2022.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Findings of a randomized, double-blind, double-dummy, multicentre phase III clinical trial that investigates the efficacy of the dapagliflozin/linagliptin fixed-dose combination (FDC) over linagliptin monotherapy in the treatment of Indian patients with type 2 diabetes mellitus (T2DM) inadequately controlled on metformin monotherapy. |
This combination therapy had shown potential to provide a more cost-effective treatment option, which is especially relevant to the Indian healthcare setting. |
The study aims to assess the individual and combined effects of dapagliflozin/linagliptin FDC in comparison to linagliptin monotherapy. |
What was learned from the study? |
Incorporation of dapagliflozin/linagliptin FDC in place of linagliptin monotherapy provides clinically significant changes in patients with T2DM as observed by the reductions in HbA1c, FPG, PPG and body weight. A statistically significant higher reduction in HbA1c (p = 0.003), FPG (p = 0.0274), PPG (p < 0.0001) and body weight (p < 0.0002) was observed in the test group compared to the reference group. |
The study establishes the superiority of the FDC over linagliptin monotherapy and presents a new therapeutic option for patients with T2DM, particularly among the Indian population who often undergo metformin monotherapy. |
Introduction
Type 2 diabetes mellitus (T2DM) is a complex, chronic condition with eight pathophysiological disturbances collectively referred to as the ‘ominous octet’ (Fig. 1). These perturbations result in insufficient glycemic control, a characteristic of T2DM, and management requires a combination of pharmacological and non-pharmacological therapies [1, 2]. Several therapeutic drug classes are available to treat T2DM when non-pharmacological therapies such as diet and exercise prove to be inadequate [2]. Metformin, a biguanide class of oral antidiabetic agent (OAD), is a first-line treatment to achieve glucose-lowering goals in patients with T2DM [3]. However, most patients with T2DM fail to maintain glycemic control with first-line OAD and require either an additional OAD or combination therapy of various OAD drug classes to reduce hyperglycemia [4].
The inability of monotherapies in aiding patients to achieve their glycemic targets could lead to an increase in macrovascular and microvascular complications as well as hypoglycemic events. Thus, combination therapies provide an aggressive and proactive approach towards treating T2DM while reducing the incidence of side effects [5]. Additionally, experts suggest that combination therapies could lead to increased patient adherence due to a decrease in pill burden [6].
Further, apart from biguanides, two classes of OADs that have proven to be produce clinically meaningful glucose-lowering effects are dipeptidyl peptidase 4 (DPP4) inhibitors and sodium-glucose co-transporter 2 (SGLT2) inhibitors. The mechanism of DPP4 inhibitors involves increasing levels of active glucagon-like peptide 1 (GLP-1) hormones. This stimulates insulin secretion, resulting in the reduction of HbA1c and fasting plasma glucose (FPG) [7, 8]. Linagliptin is a potent, efficacious, and safe DPP4 inhibitor that lowers the risk of hypoglycemia by suppressing glucagon secretion and improves prandial insulin secretion [9]. Consequently, SGLT2 inhibitors such as dapagliflozin promote urinary excretion of glucose by blocking renal glucose reabsorption that in turn aides in reduction of plasma glucose [10]. Evidence suggests it has a positive impact on the reduction of body weight, HbA1c and FPG [11, 12]. Furthermore, successful studies on combination therapy of DPP4 inhibitors and SGLT2 inhibitors such linagliptin and empagliflozin [13], teneligliptin and canagliflozin [12] as well as saxagliptin and dapagliflozin [14] provide evidence for the combination therapy to be a safe and efficacious treatment for T2DM especially in Indian patients [15]. The fixed-dose combination (FDC) is more efficacious than both the components alone in patients with T2DM [16]. Further, evidence suggests that DPP4 inhibitor and SGLT2 inhibitor FDC therapy results in a significant decrease in HbA1c in patients with T2DM for whom metformin monotherapy provides inadequate glycemic control [13, 17]. However, the SGLT2 inhibitor dapagliflozin with linagliptin as an FDC therapy has not been evaluated in patients in a double-blind, parallel-group, phase III trial before.
Thus, the aforementioned background provides a rationale for this study. It was hypothesized (H1) that FDC dapagliflozin/linagliptin would have a significant and positive effect on patients with insufficiently controlled T2DM on metformin monotherapy. On the contrary, the null hypothesis (H0) suggested that the FDC dapagliflozin/linagliptin would not have a superior effect on the study subjects in comparison to linagliptin monotherapy.
Methods
Study Details
This was a 30-week, multicenter, phase III, double-blind, double-dummy parallel-group study of once-daily dapagliflozin/linagliptin FDC compared to linagliptin single therapy in patients with inadequately controlled T2DM on metformin. The study was conducted at 16 sites across India between August 2022 and April 2023. It was in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use‐Good Clinical Practice (ICH‐GCP) and NDCT Rule 2019. The study was approved by the institutional review board of each site. Informed consent in writing was obtained from all the study subjects.
Further, the primary objective of the study was to compare the change from baseline in HbA1c at 16 weeks between FDC of dapagliflozin 10 mg and linagliptin 5 mg (test group) versus linagliptin 5 mg alone (reference group) in patients with poorly controlled T2DM with a background of metformin monotherapy. The secondary objective was to compare the change in FPG, postprandial plasma glucose (PPG) and body weight from baseline values to week 16 between test group and the reference group. It also involved evaluating the safety and tolerability of the investigational product, FDC tablets of dapagliflozin/linagliptin 10/5 mg.
Study Population
Out of the 287 patients with T2DM that were screened, 232 patients fulfilling the study selection criteria were enrolled across 16 sites in India. The inclusion criteria for the trial subjects included adult male and female patients with T2DM (age 18–65 years) with an HbA1c value between 7.5% and 10.5% (58–91 mmol/l) and an inadequate glycemic control on ≥ 1000 mg/day of metformin monotherapy for at least 28 days. The exclusion criteria included patients with known hypersensitivity to linagliptin or dapagliflozin or to any of the excipients of the investigational products; type 1 diabetes; body mass index (BMI) ≥ 40 kg/m2; FPG > 270 mg/dl; an indication of chronic kidney disease (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2); indication of liver disease (total bilirubin > 1.5 × upper limit normal (ULN), alanine transaminase (ALT)/aspartate transaminase (AST) > 2.5 × ULN, serum amylase and/or lipase > 3 × ULN).
Study Design
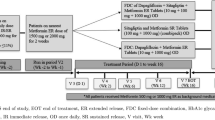
The study duration was approximately 8 months, which included a 3-month recruitment and 5-month study period (4 weeks of screening period and 16 weeks of treatment period). Further, the study design involved a 2-week run-in period post eligibility screening of the patients. During the 14-day single-blind period, all eligible patients were shifted to linagliptin 5 mg, matching the placebo of the test product (dapagliflozin/linagliptin FDC) and previous ongoing metformin treatment. Those who failed to comply with trial procedures and take at least 80% of the total doses were excluded from the study before randomization. Post the run-in period, eligible patients (n = 232) were randomized in 1:1 ratio in parallel to receive either dapagliflozin/linagliptin 10/5 mg or linagliptin 5 mg on day 1. Figure 2 provides a graphic representation of the study design. Out of the 232 eligible patients, 225 patients (test, n = 115; reference, n = 110) completed the study and 7 patients were unable to do so. Table 1 presents causes for patient attrition.
Statement of Ethics Compliance
The study was in compliance with ICH‐GCP guidelines and the NDCT Rule 2019 (India). It was approved by the ethics committee of all the 16 trial sites across India, that is, lnstitutional Ethics Committee Apex Hospitals, Pvt (ECR/380/Inst/RJ/2013/RR-19), Penta-Med Ethics Committee (ECR/357/Inst/MH/2013/RR-20), LPR Ethics Committee (ECR/751/Inst/MH/2015/RR-21), Shree Siddhivinayak Hospital Ethics Committee (ECR/1247/Inst/MH/2019), Ethics Committee of Trauma Care Hospital ECR/1623/Inst/MH/2021), Shrey Hospital Institutional Ethics Committee (ECR/1302/Inst/GJ/2019), Institutional Ethics Committee for Human Research Medical College and Hospital (ECR/287Inst/WB/2013/RR-19), Institutional Human Ethics Committee, Panimalar Medical College & Research Institute (ECR/1399/Inst/TN/2020), Sangini Hospital Ethics Committee (ECR/147/Inst/GJ/2013/RR-19), Institutional Ethics Committee Amrita Institute of Medical Sciences (ECR/129/Inst/KL/2013/RR-19), Ethics Committee of SMS Medical College and Attached Hospital (ECR/26/Inst/RJ/2013/RR-19), Institutional Ethics Committee Osmania Medical College (ECR/300/Inst/AP/2013/RR-19), Shree Siddhivinayak Maternity and Nursing Home Unity Campus (ECR/1247/Inst/MH/2019), Clinical Research Ethics Committee (ECR/194/Inst/WB/2013/RR-20), Institutional Ethics Committee, Meenakshi Mission Hospital and Research Centre (ECR/398/Inst/TN/2013/RR-19), and Help Hospitals Pvt. Ltd (ECR/1356/Inst/AP/2020). The study involves human subjects and informed consent was obtained from all 232 patients involved in the study.
Efficacy Outcome Measures
The primary endpoint of the study was the change in HbA1c from baseline to week 16 for dapagliflozin/linagliptin 10/5 mg and linagliptin 5 mg. The secondary endpoint included changes in FPG, PPG and body weight from baseline to week 16 for the same regimen. A statistically significant reduction in primary and secondary endpoints is observed in the test group compared to the reference group.
Safety Outcome Measures
The adverse events (AEs; coded using Medical Dictionary for Regulatory Authorities [MedDRA], version 25.0) were recorded post study drug administration and were termed treatment emergent adverse events (TEAEs). The TEAEs were categorized by system organ class (SOC) and were summarized on the basis of severity, action taken, relatedness and outcome. The incidence of TEAEs by SOCs included general disorders and administration; pain; pyrexia; infections and infestations; nasopharyngitis; upper respiratory tract infection; urinary tract infection; investigations; decreased haemoglobin; decreased platelet count; arthralgia; musculoskeletal and connective tissue; back pain; pain in extremity; nervous system disorders; headache; cough; respiratory, thoracic and mediastina. Twelve-lead ECG and haematology and urinary parameters were measured at visits 1 and 6. Biochemical parameters were measured at visits 1, 3 and 6; vital signs and a physical examination were carried out at all six visits.
Statistical Analysis
Analysis for the efficacy and safety was performed by per protocol set (PPS) and intention-to-treat (ITT) analysis, respectively. The between-group difference, that is, the effect size in HbA1c change from baseline, was 0.4%, and the standard deviation (SD) was 1%. The required sample size in two groups with 15% dropout was 232; without a 15% dropout it was 196 subjects (98 in each group) (supplementary material Table S1).
The primary and secondary endpoints were analysed by an analysis of covariance (ANCOVA) model with treatment and stratification factor HbA1c level ≤ 9.0% and > 9.0% as factor and baseline as covariate. The ANCOVA model was used to derive a least-squares estimate of the treatment difference with a 95% confidence interval (CI) and a corresponding two-sided p value. Furthermore, the two-sided 95% CI for the mean change within each treatment group was calculated. A full analysis set was used to evaluate the primary and secondary efficacy endpoints. The p value derived from the ANCOVA model or 95% CI of treatment difference least-squares estimate means would be the proof of superiority.
Results
Patient Disposition, Demographic and Baseline Characteristics
A total of 287 patients with T2DM were screened, of which 55 were ineligible and 232 were eligible for the study as a result of the exclusion and inclusion criteria respectively. All 232 patients were enrolled in the study and randomized. Seven patients discontinued the study. Out of the study subjects, 120 (51.7%) were male and 112 (48.3%) were female. At baseline, the mean age and weight of subjects in the test group were 49.5 ± 9.67 years and 66.0 ± 10.81 kg respectively; those in the reference group were 49.8 ± 9.69 years and 65.7 ± 11.04 kg respectively. The mean BMI was 25.2 ± 3.72 kg/m2 in the test group and 25.3 ± 3.36 kg/m2 in the reference group. The mean duration of T2DM was 8.7 ± 8.21 months (Table 2). The mean HbA1c was 8.71 ± 0.76% in the test group and 8.75 ± 0.86% in the reference group (Table 3). All patient demographics and baseline values were comparable and similar between both groups.
Change in HbA1c
Both treatment groups shared comparable baseline HbA1c (p = 0.7101). The mean HbA1C (%) was 8.71 ± 0.756 in the test group and 8.75 ± 0.863 in the reference group. After completion of the study duration at 16 weeks, both treatment groups showed a significant decrease in HbA1c (%) (p < 0.0001). The mean HbA1c in the test group was 7.43 ± 0.816 (with a mean reduction of − 1.28 ± 0.857) and 7.92 ± 1.164 (with a mean reduction of − 0.83 ± 1.229) in the reference group (Fig. 3). However, the test group exhibited a larger and statistically significant reduction in HbA1c (p = 0.0003). Additionally, the least-squares mean change (LSM) in HbA1c (%) from baseline was − 1.3 in the test group compared to − 0.8 in the reference group. The difference in the LSM change in HbA1c between the test and reference was 0.5 and the 95% confidence interval was − 0.7360, − 0.2251.
Figure 4 provides an insight into the percentage of patients with an HbA1c value of < 7.0 in week 16 (EOS). At week 16, 30 (25.86%) patients in the test group and 15 (12.93%) patients in the reference group experienced a reduction in HbA1c to below 7.0%.
Change in FPG, PPG and Body Weight
The baseline mean values of FPG (mg/dl), PPG (mg/dl) and body weight (kg) were comparable between the treatment groups (p = 0.5313; p = 0.9983; p = 0.7002 respectively). At week 16 (EOS), the test group exhibited a larger reduction in all three variables compared to the reference group. Furthermore, the mean reduction for FPG, PPG and body weight for each treatment group was statistically significant. However, statistically the magnitude of reduction for the test group was significantly higher than the reference group (Table 4).
Safety and Tolerability Measures
Overall, the incidence of TEAEs was lower in patients receiving the dapagliflozin/linagliptin treatment (7 subjects; 6%) compared to the single therapy, linagliptin (12 subjects; 10.3%). A total of 19 TEAEs from 19 subjects (8.2%) were reported during the study. Out of the 19 TEAEs, 17 AEs were mild (grade 1) and 2 AEs were moderate (grade 2) in intensity (Table 5). There were no SAEs reported in the study. Further, with respect to causality, one event was reported in each treatment group that was considered to be related to the investigational product (Table 6).
Discussion
This double-blind, parallel-group study trial was the first randomized trial to evaluate the effect of dapagliflozin/linagliptin FDC in patients with T2DM, especially Indian patients. A statistically significant higher reduction in HbA1c (p = 0.003), FPG (p = 0.0274), PPG (p < 0.0001) and body weight (p < 0.0002) was observed in the test group compared to the reference group. Furthermore, the test group had double the number of patients experiencing a reduction in HbA1c to below 7.0% (53 mmol/l), compared to the reference group. Thus, our results provide evidence for dapagliflozin/linagliptin FDC affording better glycemic control than linagliptin monotherapy in patients with T2DM and inadequate glycemic control with a background of metformin therapy.
The results for HbA1c (mean reduction of 1.28% in 16 weeks) are in line with results of previous, similarly designed trials carried out on SGLT2 inhibitor-linagliptin FDC [13], supporting the clinical relevance of study results. The former exhibited a lower reduction in HbA1c (1.14% in 24 weeks) compared to our study. The latter could be a result of the lower body weight and a higher baseline HbA1c value of study subjects in this trial as well as racial differences. Further, another trial [12] on DPP4/SGLT2 inhibitor FDC (canagliflozin/teneligliptin) highlighted a lower reduction in HbA1c (0.88%) compared to both this study as well as the trial reported by Kawamori et al. [13]. However, as per the American Diabetes Association and European Association for Study of Diabetes guidelines, HbA1c level should be ≤ 7.0% (≤ 53 mmol/l) whereas the minimum threshold for HbA1c in this study was 7.5% (58 mmol/l). Thus, a higher reduction in HbA1c could be a result of the same. Moreover, in comparison to the empagliflozin/linagliptin trial reported by Kawamori et al. [13] the duration of the trial was much shorter (30 weeks and 52 weeks, respectively). Additionally, the SGLT2 inhibitor dose was significantly lower (dapagliflozin 5 mg and empagliflozin 25 mg). This suggests that to attain the ideal HbA1c level of ≤ 7.0% (≤ 53 mmol/l), a trial with a longer duration and higher dose of dapagliflozin should be carried out.
Furthermore, as mentioned above, a decrease in FPG, PPG and body weight, characteristic of SGLT2 inhibitors, was also observed in this trial [10]. Additionally, the dapagliflozin/linagliptin FDC therapy enhanced metabolic control without causing an increase in glucagon. This could be due to the opposing effects of DPP4 inhibitors and SGLT2 inhibitors on glucagon levels, FPG and PPG. The former reduces glucagon levels and either increases or has no effect on FPG [18, 19] whereas the latter increases glucagon levels while simultaneously lowering FPG and PPG [20,21,22,23].
Dapagliflozin/linagliptin FDC’s safety profile is consistent with those of the individual drug components, that is, no new safety signals and AEs were observed that were not expected from the individual components of the FDC [6, 24]. Overall, dapagliflozin/linagliptin FDC was well tolerated. Thus, all the results mentioned above from this trial substantiate that the FDC is a compelling and effective therapeutic option in comparison to linagliptin monotherapy for patients with insufficiently controlled T2DM with a history of metformin monotherapy.
Despite this study enrolling only Indian patients with T2DM, the results are strengthened by data from other multinational trials as well as the double-blind, double-dummy, parallel-group study design with a 2-week run-in period. The trial very clearly reflects the clinical setting in which a second-line combination therapy of DPP4 inhibitors and SGLT2 inhibitors is used to treat patients with T2DM which was poorly controlled by metformin. The statistical power for the primary and secondary endpoints as well as the completion rates (> 90%) were high throughout the 16 weeks of the study period. Conversely, it is essential to investigate the low BMI observed among the patients in the study. Indian patients with T2DM have a unique profile, that is, involving a lower BMI, in comparison to their Western counterparts. The discrepancy could be attributed to the presence of higher levels of visceral fat or brown adipose tissue which are associated with insulin resistance and increased risk of T2DM. This highlights the need to explore these distinctive characteristics further.
Conclusion
Incorporation of dapagliflozin/linagliptin FDC in place of linagliptin monotherapy provides clinically significant changes in patients with T2DM as observed by the reductions in HbA1c, FPG, PPG and body weight. The efficacy, tolerability and safety of dapagliflozin/linagliptin FDC make it a key contender to treat patients with a background of metformin monotherapy.
Data Availability
The datasets generated and analyzed during the study are available from the corresponding author on reasonable request.
References
Khurana L, Durand EM, Gary ST, et al. Mechanisms for improving diabetes patient–provider communication through optimal use of e-clinical technologies. Patient Prefer Adherence. 2019;13:981–92.
Davies MJ, Aroda VR, Collins BS, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45:2753–86.
Brunetti L. Management of type-2 diabetes mellitus in adults; focus on individualizing non-insulin therapies. P T. 2012;37:687–96.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes 2015, a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2014;38:140–9.
Kalra S, Das A, Priya G, et al. Fixed-dose combination in management of type 2 diabetes mellitus: expert opinion from an international panel. J Family Med Primary Care. 2020;9:5450.
Del Prato S, Felton A-M, Munro N, Nesto R, Zimmet P, Zinman B. Improving glucose management: ten steps to get more patients with type 2 diabetes to glycaemic goal. Int J Clin Pract. 2005;59:1345–55. https://doi.org/10.1111/j.1742-1241.2005.00674.x.
Chen XW, He ZX, Zhou ZW, et al. Clinical pharmacology of dipeptidyl peptidase 4 inhibitors indicated for the treatment of type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015;15(42):999–1024.
Del Prato S, Taskinen MR, Owens DR, et al. Efficacy and safety of linagliptin in subjects with type 2 diabetes mellitus and poor glycemic control: pooled analysis of data from three placebo-controlled phase III trials. J Diabetes Complicat. 2013;27:274–9.
Ning G, Bandgar T, Hehnke U, Lee J, Chan JCN. Efficacy and safety of linagliptin in 2681 Asian patients stratified by age, obesity, and renal function: a pooled analysis of randomized clinical trials. Adv Ther. 2017;17(34):2150–62.
Levine MJ. Empagliflozin for type 2 diabetes mellitus: an overview of phase 3 clinical trials. Curr Diabetes Rev. 2017;25(13):405–23.
Araki E, Yukio T, Tanaka Y, et al. Long-term treatment with empagliflozin as add-on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:665–74.
Kadowaki T, Inagaki N, Kondo K, et al. Efficacy and safety of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: results of a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017;31(19):874–82.
Kawamori R, Haneda M, Suzaki K, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2018;1(20):2200–9.
Zhuang Y, Song J, Ying M, Li M. Efficacy and safety of dapagliflozin plus saxagliptin vs monotherapy as added to metformin in patients with type 2 diabetes. Medicine. 2020;24(99):e21409.
Chadha M, Das AK, Deb P, et al. Expert opinion: optimum clinical approach to combination-use of SGLT2i + DPP4i in the Indian diabetes setting. Diabetes Ther. 2022;25(13):1097–114.
DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;12(38):384–93.
Tinahones FJ, Gallwitz B, Nordaby M, et al. Linagliptin as add-on to empagliflozin and metformin in patients with type 2 diabetes: two 24-week randomized, double-blind, double-dummy, parallel-group trials. Diabetes, Obes Metab. 2017;19:266–74.
D’Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab. 2011;8(13):126–32.
Rauch T, Graefe-Mody U, Deacon CF, et al. Linagliptin increases incretin levels, lowers glucagon, and improves glycemic control in type 2 diabetes mellitus. Diabetes Ther. 2012;3:10.
Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Investig. 2014;27(124):499–508.
Forst T, Falk A, Andersen G, et al. Effects on α- and β-cell function of sequentially adding empagliflozin and linagliptin to therapy in people with type 2 diabetes previously receiving metformin: an exploratory mechanistic study. Diabetes Obes Metab. 2017;10(19):489–95.
Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Investig. 2014;27(124):509–14.
Muscelli E, Brenno A, Barsotti E, et al. Metabolic consequences of acute and chronic empagliflozin administration in treatment-naive and metformin pretreated patients with type 2 diabetes. Diabetologia. 2015;59:700–8.
Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I–III clinical trials. Adv Ther. 2017;19(34):1707–26.
Carbon Health. The ominous octet. 2023. https://education.steady.health/hc/en-us/articles/360052231513-The-Ominous-Octet. Accessed 27 Aug 2023.
BioRender. app.biorender.com. 2023 https://app.biorender.com/illustrations/64ec62a811c68a623ed020e8. Accessed 28 Aug 2023.
Acknowledgements
Medical Writing.
Medical writing assistance in the preparation of this article, that is, the original draft, final draft and all the subsequent revised editions was provided by Aditi Jain from Deanery of Biomedical Sciences, The University of Edinburgh. There was no funding involved for the assistance.
Funding
Sponsorship for this study and Rapid Service Fee is funded by Alkem Laboratories Ltd.
Author information
Authors and Affiliations
Contributions
All authors, that is, Aditi Jain, Dr. Abhay Vispute, Dr. Amol Dange, Dr. Arindam Naskar, Dr. Asish Mondal, Dr. B. Vivekanand, Dr. Balram Sharma, Dr. Deepak Varade, Dr. Dhaiwat Shukla, Dr. Girish Bhatia, Dr. Harshal Chaudhari, Dr. K. Ram Babu, Dr. Onkar Gavali, Dr. Sanket Sorate, Dr. Shaishav Bhanushali, Dr. Vaibhav Kothari, Dr. Vipul Khandelwal, Dr. Akhilesh Sharma, Dr. Roshan Pawar, Dr. Mayur Mayabhate, Dr. Vinayaka Shahavi, Dr. Aashishsingh Rajput and Mukesh Jaiswal contributed to the study design, conception and data collection; reviewed and commented on the original manuscript draft; read and approved the final manuscript. Writing of the manuscript, that is, the original draft preparation and editing; material preparation and formal analysis was carried out by Aditi Jain.
Corresponding author
Ethics declarations
Conflict of Interest
Aditi Jain, Dr. Abhay Vispute, Dr. Amol Dange, Dr. Arindam Naskar, Dr. Asish Mondal, Dr. B. Vivekanand, Dr. Balram Sharma, Dr. Deepak Varade, Dr. Dhaiwat Shukla, Dr. Girish Bhatia, Dr. Harshal Chaudhari, Dr. K. Ram Babu, Dr. Onkar Gavali, Dr. Sanket Sorate, Dr. Shaishav Bhanushali, Dr. Vaibhav Kothari, Dr. Vipul Khandelwal, Dr. Akhilesh Sharma, Dr. Roshan Pawar, Dr. Mayur Mayabhate, Dr. Vinayaka Shahavi, Dr. Aashishsingh Rajput, and Mukesh Jaiswal have no competing interests. The authors have no financial or non-financial interest to declare. No funding was received for the preparation of this manuscript.
Ethical Approval
The study was in compliance with ICH‐GCP guidelines and the NDCT Rule 2019 (India). It was approved by the ethics committee of all the 16 trial sites across India, that is, lnstitutional Ethics Committee Apex Hospitals, Pvt (ECR/380/Inst/RJ/2013/RR-19), Penta-Med Ethics Committee (ECR/357/Inst/MH/2013/RR-20), LPR Ethics Committee (ECR/751/Inst/MH/2015/RR-21), Shree Siddhivinayak Hospital Ethics Committee (ECR/1247/Inst/MH/2019), Ethics Committee of Trauma Care Hospital ECR/1623/Inst/MH/2021), Shrey Hospital Institutional Ethics Committee (ECR/1302/Inst/GJ/2019), Institutional Ethics Committee for Human Research Medical College and Hospital (ECR/287Inst/WB/2013/RR-19), Institutional Human Ethics Committee, Panimalar Medical College & Research Institute (ECR/1399/Inst/TN/2020), Sangini Hospital Ethics Committee (ECR/147/Inst/GJ/2013/RR-19), Institutional Ethics Committee Amrita Institute of Medical Sciences (ECR/129/Inst/KL/2013/RR-19), Ethics Committee of SMS Medical College and Attached Hospital (ECR/26/Inst/RJ/2013/RR-19), Institutional Ethics Committee Osmania Medical College (ECR/300/Inst/AP/2013/RR-19), Shree Siddhivinayak Maternity and Nursing Home Unity Campus (ECR/1247/Inst/MH/2019), Clinical Research Ethics Committee (ECR/194/Inst/WB/2013/RR-20), Institutional Ethics Committee, Meenakshi Mission Hospital and Research Centre (ECR/398/Inst/TN/2013/RR-19), and Help Hospitals Pvt. Ltd (ECR/1356/Inst/AP/2020). The study involves human subjects and informed consent was obtained from all 232 patients involved in the study. Consent to publication is not applicable to this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jain, A., Vispute, A., Dange, A. et al. A Randomized, Double-Blind, Parallel-Group Phase III Trial Investigating the Glycemic Efficacy and Safety Profile of Fixed-Dose Combination Dapagliflozin and Linagliptin Over Linagliptin Monotherapy in Patients with Inadequately Controlled Type 2 Diabetes with Metformin. Diabetes Ther 15, 215–227 (2024). https://doi.org/10.1007/s13300-023-01504-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01504-3