Abstract
Introduction
Diabetic kidney disease (DKD) has become the leading cause of chronic kidney disease and end-stage renal failure in most developed and many developing countries. Strategies aimed at identifying potential modifiable risk factors for DKD are urgently needed. Here, we investigated the association between clusters of body fat and nutritional parameters with DKD in adults with type 2 diabetes mellitus (T2DM).
Methods
This was a cross-sectional study of 184 participants with T2DM. Biochemical parameters including fasting blood glucose, hemoglobin A1c, hemoglobin, albumin, creatinine, and urinary albumin-to-creatinine ratio (UACR) were measured. The data for percentage of body fat mass (PBF), visceral fat area (VFA), phase angle at 50 kHz (PA50), and body cell mass (BCM) were obtained by bioelectrical impedance analysis (BIA). DKD was diagnosed by UACR and estimated glomerular filtration rate. Factor analysis was used for dimensionality reduction clustering among variables. The association of clusters with the presence of DKD was assessed using binary logistic regression analysis.
Results
Factor analysis identified two clusters which were interpreted as a body fat cluster with positive loadings of VFA, body mass index, waist circumstance, and PBF and a nutritional parameters cluster with positive loadings of PA50, hemoglobin, BCM, and albumin. Participants were divided into the four groups based on the sex-specific cutoff value (median) of each cluster score calculated using the cluster weights and the original variable values. Only participants with high body fat and poor nutritional parameters (OR 3.43, 95% CI 1.25–9.42) were associated with increased odds of having DKD.
Conclusion
Body fat and nutritional parameters were strongly associated with and considerably contributed to the presence of DKD, suggesting that body fat and nutrition might be promising markers representing metabolic state in pathogenesis of DKD and clinical utility of BIA might provide valuable recommendations to patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Diabetic kidney disease (DKD) develops in approximately 40% of patients with diabetes and has become the leading cause of chronic kidney disease. Strategies aimed at identifying potential modifiable risk factors for DKD are urgently needed. |
Factor analysis was applied to investigate whether body composition and biochemical parameters were associated with DKD. |
What was learned from the study? |
Two clusters interpreted as body fat and nutritional parameters were identified in the study population. Subjects with diabetes with high body fat and poor nutritional parameters had increased odds of DKD. |
Body fat and nutrition might be promising markers that represent metabolic state in pathogenesis of DKD and clinical utility of bioelectrical impedance analysis might provide valuable recommendations to patients with T2DM. |
Introduction
The prevalence of diabetes mellitus among Chinese adults is approximately 11.2% [1]. Diabetic kidney disease (DKD) develops in 20–40% of patients with diabetes [2] and has become the leading cause of chronic kidney disease (CKD) in China [3]. A meta-analysis of observational studies demonstrated that the overall pooled prevalence of DKD among Chinese patients with type 2 diabetes mellitus (T2DM) was 21.8% [4], indicating that strategies aimed at identifying potential modifiable risk factors for DKD are urgently needed to screening and management of DKD. However, currently the pathogenesis of DKD remains incompletely understood.
To date, risk factors for DKD are conceptually classified as susceptibility factors (age, sex, race, etc.), initiation factors (hyperglycemia and acute kidney injury), and progression factors (hypertension, obesity, etc.) [5]. A DKD risk prediction model consisting of nine risk factors, namely age, body mass index (BMI), smoking, diabetic retinopathy (DR), hemoglobin A1c (HbA1c), systolic blood pressure (BP), high-density lipoprotein (HDL), triglyceride (TG), and urinary albumin-to-creatinine ratio (UACR), was established and validated for detecting patients at high risk of DKD with a sensitivity of 0.85 and a specificity of 0.68 [6].
In recent years, there has been emerging interest in assessing body composition parameters using bioimpedance analysis, which estimate the body composition based on different electricity flows in different media such as water and fat [7]. Changes in fat distribution in adults may contribute to the increased risk of T2DM. Moreover, lifestyle, pharmacological, and surgical interventions that lower visceral fat may be of benefit for patients with T2DM [8]. Body composition measures were strongly associated with the presence and severity of DR, which is one of the diabetic microvascular complications besides DKD [9]. Specifically, perirenal fat thickness was significantly associated with the risk for development of DKD [10]. Notably, the body composition parameters also include phase angle at 50 kHz (PA50), and body cell mass (BCM), which may better reflect a person’s cellular integrity and nutritional status. However, few studies have applied these nutritional parameters in persons with diabetes.
In this study, we aimed to investigate the associations of body fat and nutritional parameters assessed by body composition measurements with the presence of DKD in patients with T2DM.
Methods
Ethics Approval and Consent to Participate
This study adhered to the principles of the Declaration of Helsinki of 1964 and its later amendments and the protocols were approved by the ethics committee of Nanjing Medical University (Approval No. 2019KY097). The ethics clearance, information sheet, and consent form were approved by the faculty institutional review board. Study participants were outpatients visiting the department of Nephrology and Endocrinology, the Second Affiliated Hospital of Nanjing Medical University. We obtained written informed consent from all participants. No identifying information was included in the manuscript.
Study Participants
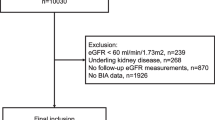
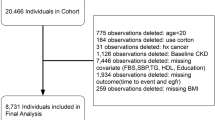
The present cross-sectional study recruited participants aged ≥ 18 years with T2DM who underwent body composition measurements between January 2020 and December 2020 in a consecutive manner. T2DM was confirmed using either one of the following criteria: fasting plasma glucose ≥ 126 mg/dL, random glucose ≥ 200 mg/dL, or HbA1c ≥ 6.5% according to American Diabetes Association 2018 criteria [11]. DKD was diagnosed as urinary albumin-to-creatinine (UACR) ≥ 30 mg/g or estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 in the absence of signs or symptoms of other primary causes of kidney damage [12]. eGFR was calculated using the chronic kidney disease epidemiology collaboration equation (CKD-EPI equation, http://www.nkdep.nih.gov). The participants with unreliable body composition measurement data, lacking urinary albumin creatinine or serum creatinine were excluded from this study. Moreover, patients with nondiabetic kidney disease (NDKD), acute inflammatory diseases, acute kidney injury, systemic diseases, malignant neoplasm, endocrine disorders other than T2DM, or having received kidney replacement therapy were excluded (Fig. 1).
Clinical Examination and Interview
Blood and morning urine samples were collected from all participants who had fasted overnight. The biochemical parameters including fasting blood glucose (FBG), HbA1c, hemoglobin, albumin, TG, total cholesterol (TC), low-density lipoprotein (LDL), HDL, UACR, serum creatinine, and uric acid were tested. Smoke and alcohol consumption were categorized on the basis of the participants’ responses in a standard questionnaire. Height, weight, waist circumference (WC), BP, and heart rate (HR) were measured by a trained nurse. An arm electronic sphygmomanometer was applied to measure HR and BP. The average of two separate measurements of systolic and diastolic BP was recorded. A third measurement was be taken if a BP difference ≥ 10 mmHg for systolic BP or ≥ 5 mmHg for diastolic BP occurred with the two closest BP measurements averaged and recorded [13]. Hypertension was diagnosed for those with systolic BP > 140 mmHg or diastolic BP > 90 mmHg or on antihypertension medication [14]. Coronary artery disease (CAD) and diabetic retinopathy (DR) were registered according to previous medical record. Height, WC, and weight were measured using an adjustable and wall-mounted tape, a 151-cm medical tape, and a calibrated digital weight scale (Omron Karada Scan Body Composition Scale HBF-375; Omron, Osaka, Japan), respectively. BMI was calculated by weight in kilograms divided by the square of height in meters.
Measurement of Body Composition Parameters
Bioelectrical impedance analysis (BIA) was applied to evaluate body composition using InBody S10 (InBody Japan, Tokyo, Japan) as previously described [9]. Patients that have any medical electrical devices were not subjected to use this equipment. Study populations were not allowed to eat, exercise, take a bath, or shower prior to testing. Measurement was performed in a lying posture after urination or excretion before midday under normal temperature conditions (20–25 °C). The examinee maintained a lying posture for about 10–15 min before and during the test with arms spread naturally to a 15° angle away from the trunk and legs spread to shoulder width. After the electrodes (touch type) were connected, information of weight, height, age, and gender were input and the test was started. The data for body fat mass (kg), visceral fat area (VFA, cm2), PA50 (degree), and BCM (kg) were obtained. Percentage of body fat mass (PBF, %) was calculated by dividing body fat mass in kg by body weight in kg.
Statistical Analysis
We used the SPSS 25 software package for data analysis. Continuous variables were described using mean ± standard deviation (SD) or median (25th and 75th percentiles), depending on whether or not the data were normally distributed (assessed by the Shapiro–Wilk test). Categorical variables are reported as proportions. Student t test or Mann–Whitney U test was used to compare continuous variables between two groups. Analysis of variance (ANOVA) or the Kruskal–Wallis test was used to compare among three or more groups. χ2 tests were used to compare proportion variables.
Factor analysis was used for dimensionality reduction clustering among variables to distill complex information from multiple factors into summary scores representing distinct dimensions [15, 16]. Briefly, model fit was tested by the Kaiser–Meyer–Olkin measure of sampling adequacy. The calculated value of 0.606 was regarded as an indicator of good model fit. Bartlett’s test for sphericity was significant with a P value < 0.001 indicating significant relationships between the variables. To determine the number of factors to extract, we initially performed principal components analysis and examined the number of eigenvalues > 1 and the scree plot; we also conducted parallel analysis. Varimax rotation was then performed with identification of variables comprising a cluster based on loadings greater than 0.5. To derive factor scores, we used the regression scoring method, which created standardized scores for each cluster. These scores represented the subjects’ predicted values for each cluster and were calculated using the cluster weights and the original variable values. Names for each cluster were created according to the hypothesized physiology of the most highly loading factors.
Associations between cluster scores and clinical characteristics were examined by Spearman correlation test. Binary logistic regression model was applied to estimate the associations of different group with the presence of DKD. Model 1 was unadjusted. Model 2 was adjusted for age and sex. Model 3 was further adjusted for smoking, diabetes duration, systolic BP, diastolic BP, FBG, TC, and TG. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
Results
Table 1 summarizes the clinical characteristics, biochemistry results, and body composition parameters at study entry. We recruited 184 participants (64.67% male) with a median age of 57 years. Ninety-three (50.54%) of the participants had a history of hypertension and the median duration of diabetes was 68.50 months. The median eGFR was 100.13 ml/min/1.73 m2. Sixteen participants had eGFR lower than 60 ml/min/1.73m2. Eighty-six (46.74%) participants were diagnosed with DKD. The participants had a mean BMI of 25.65 ± 3.40 kg/m2 and a median WC of 88.35 (82.13, 95.83) cm. The median hemoglobin was 145.00 (131.25, 157.00) g/L and median albumin was 43.15 (39.95, 46.48) g/L. Body composition measurements showed that mean PBF was 28.07 ± 6.77%, median VFA was 87.85 (69.23, 110.63) cm2, mean PA50 was 5.63 ± 0.89°, and mean BCM was 33.65 ± 6.12 kg. Female subjects had lower levels of hemoglobin, PA50, BCM, and WC and higher levels of PBF and VFA compared to male subjects. The presence of DKD was similar among female and male subjects.
Table 2 summarizes cluster analysis results for the total group of 184 participants. A model with two distinct factors was chosen and there was a reduction in variable number from the original eight to two composite clusters. These two clusters taken together explained about 72.71% of the variance in the original data (cluster 1, 42.48%; cluster 2, 30.23%). These clusters were interpreted as a body fat cluster with positive loadings of VFA, BMI, WC, and PBF and a nutritional parameters cluster with positive loadings of PA50, hemoglobin, BCM, and albumin.
Table 3 shows the univariate analysis of the relationships between scores of body fat cluster or nutritional parameters cluster and characteristics of the T2DM. We found significant correlation between body fat cluster and hypertension in all participants with T2DM. However, we found no significant correlation between body fat cluster and gender, age, smoking, duration of diabetes, glucose and lipid levels, UACR or stages of DKD in participants with DKD. In the meantime, nutritional parameters cluster correlated with gender, age, smoking, duration of diabetes, HDL, UACR and stages of DKD in participants with DKD. Also, there were no significant correlation between nutritional parameters cluster and glucose level in participants with DKD.
Participants were then divided into four groups based on the sex-specific cutoff value (median) of each cluster score. The median for cluster 1 (body fat) score is 0.08 for female participants and − 0.16 for male participants, while median for cluster 2 (nutritional parameters) the score is − 0.84 for female participants and 0.64 for male participants. As shown in Fig. 2, group A represents participants with low body fat and good nutritional parameters, group B represents participants with low body fat and poor nutritional parameters, group C represents participants with high body fat and good nutritional parameters, and group D represents participants with high body fat and poor nutritional parameters.
Four groups of participants divided on the basis of sex-specific cutoff value (median) of each cluster score. A, low body fat and good nutritional parameters; B, low body fat and poor nutritional parameters; C, high body fat and good nutritional parameters; D, high body fat and poor nutritional parameters
Table 4 shows the comparison of participants’ characteristics across different groups according to the two clusters. Participants with poor nutritional parameters (group B and D) were more likely to have been older at recruitment, to have decreased eGFR (< 90 ml/min/1.73 m2), and increased UACR (> 30 mg/g). Participants with high body fat and good nutritional parameters (group C) were more likely to have shorter history of diabetes, and higher serum uric acid level. Notably, participants with low body fat and good nutritional parameters (group A) were less commonly to have hypertension and DKD. Characteristics of male sex, heart rate, fast glucose level, HbA1c, and TC were similar among the four groups.
The association of clusters with the presence of DKD was assessed using binary logistic regression analysis (Table 5). As compared with participants with low body fat and good nutritional parameters (group A), high body fat and good nutritional parameters (group C) was not associated with having DKD. In contrast, both low body fat and poor nutritional parameters (group B, OR 2.90, 95% CI 1.17–7.13) and high body fat and poor nutritional parameters (group D, OR 4.09, 95% CI 1.59–10.53) were associated with increased odds of having DKD after adjustment for age and sex. After further adjustment for smoking, diabetes duration, systolic BP, diastolic BP, fast glucose, TC, and TG, only high body fat and poor nutritional parameters (group D, OR 3.43, 95% CI 1.25–9.42) were associated with increased odds of having DKD.
Discussion
In this study, we have identified two clusters by factor analysis including body fat cluster consisting of VFA, BMI, WC, PBF, and nutritional parameters cluster consisting of PA50, hemoglobin, BCM, and albumin. After dividing participants into the four groups based on the cluster scores, we have demonstrated that those with poor nutritional parameters were more likely to have decreased eGFR and increased UACR, whereas participants with low body fat and good nutritional parameters were less commonly to have DKD. Our noteworthy finding is that particular high body fat and poor nutritional parameters were significantly associated with increased odds of having DKD in this study population. We therefore suggest that body fat and nutritional parameters might be promising markers representing metabolic state and signify their importance in the pathogenesis of DKD. Clinical utility of BIA might provide valuable recommendations to patients with T2DM.
DKD risk factors are multifactorial and complex, involving genetic and environmental factors, which mainly derived from cohort studies with different sample sizes [5, 6]. Results of these studies were not completely consistent, and the only proven primary prevention interventions for DKD are merely blood glucose and blood pressure control [12]. For the first time to our knowledge, this study has investigated the relation of a composite indicator of body fat and nutritional parameters to the presence of DKD in patients with T2DM. Nutritional assessment includes several categories: BMI, body composition (e.g., PBF, PA50), blood biomarkers (e.g., albumin, hemoglobin), nutritional screening tool (Mini Nutritional Assessment-Short Form), anthropometric measurements (e.g., weight, WC), and dietary assessment (e.g., food and energy intake) [17]. Optimal, universal, and reliable nutritional status screening questionnaires and equations are still lacking. Body composition measure and biochemical test are precise and reliable as well as inexpensive and simple [18]. Interestingly, dimensionality reduction clustering by factor analysis distinguished two clusters, which are integrated as cluster of body fat (VFA, BMI, WC, and PBF) and cluster of nutritional parameters (PA50, hemoglobin, BCM, and albumin). A previous cross-sectional study performed in subjects with T2DM and age- and BMI-matched control subjects indicated that phase angle was a promising measurement for assessing catabolic state in people with diabetes [19]. Phase angle reflecting the poor nutritional status was associated with the lean tissue index, hemoglobin level, albumin level, and eGFR in patients with diabetic CKD stage 5 [20]. The association between scores of body fat cluster or nutritional parameters cluster and characteristics of the T2DM suggested several confounding factors, such as hypertension, gender, age, smoking, duration of diabetes, and stages of DKD. However, the association of body fat and nutrition with DKD was still reliable after adjustment of several possible confounding factors and coexisting comorbidities.
Findings from this study demonstrating that high body fat and poor nutritional parameters are strongly associated with the presence of DKD are pathophysiologically plausible. Diet insecurity may increase the risk and progression of diabetes complications through nutritional pathways and promising food security interventions have demonstrated positive impacts on diabetes outcomes [21]. The significance of the relationship between adipose tissue and T2DM has long been cemented. Distribution of adipose tissue rather than the total amount is more crucial in the development of vascular complications in Asian patients with T2DM [22]. Perirenal fat thickness significantly raised the risk for CKD in patients with diabetes [10]. Visceral adiposity and abdominal obesity are more closely associated with DKD [23, 24]. However, our knowledge of the multifaceted role of fat in disease progression is evolving and expanding [25, 26]. First, fat or adipose tissue has been far beyond a simple depot for energy storage. Reports have highlighted their endocrine signaling in insulin resistance [27]. A multitude of bioactive compounds, including adipokines, cytokines, and other lipid biomolecules, are actively secreted by adipose tissue, which have potential impacts on metabolism [28]. Second, adipose tissue comprises a multitude of different cell types besides adipocytes and preadipocytes, such as fibroblasts, endothelial cells, and immune cells, which have distinct contributions [29]. Third, different compartments of fat, including subcutaneous adipose depot, visceral adipose depot, and specifically adipose in and around metabolic organs, such as pancreas, skeletal muscle, vasculature, and kidney, have received most attention with regard to metabolic diseases [30]. Moreover, the significant distinctions between brown, beige, white, and pink adipocytes and the plasticity of adipose tissues impact the pathogenesis of various diseases [31]. We still do not have comprehensive understandings of the connections between fat and T2DM. There are even more large holes in our knowledge of fat and DKD.
This study has important clinical implications. To date, several medications have been applied to attenuate the progression of DKD [32]. However, blockade of renin–angiotensin system was not associated with attenuation of long-term risk of GFR decline [33], especially among patients with advanced CKD (eGFR < 30 ml/min/1.73 m2) [34]. Sodium–glucose cotransporter 2 (SGLT2) inhibitors prevented progression of CKD in patients with diabetes [35, 36], and evidence suggested that glucagon-like peptide 1 receptor agonists (GLP-1RA) had benefits for the kidney [37]. Mineralocorticoid receptor antagonists (MRA) have proven to be effective in reducing kidney disease progression in patients with diabetes [38]. However, the aforementioned actions of SGLT2 inhibitors, GLP-1RA, and MRA were largely consistent with improved glucose and BP control, as well as significant weight loss and improving inflammation and dysmetabolism. Medications specifically to prevent and treat DKD are quite limited; management strategies usually include lifestyle modifications, including diet interventions, physical activities, and weight control. Studies have evaluated the role of nutrition interventions in the management of T2DM and the positive results are predictable. A clinically recommended low-protein diet is expected to retard renal function decline in DKD [39]. The diet prescription may include nutrient types (e.g., carbohydrate, fat, micronutrients, vitamins), energy, and glycemic index, which should be tailored to meet the needs and characteristics of each patient [40].
We acknowledge several limitations in this study. First, the cross-sectional design used here limited the time inference of the predictor and outcomes. Prospective studies are needed to evaluate the sequence of these associations.
Second, the diagnosis of DKD was based on UACR and eGFR, which is less accurate than kidney pathology. However, renal biopsy is still at risk of missing atypical DKD, and its indication in patients with diabetes is controversial [41, 42]. The typical presentation of DKD is considered to include a long-standing duration of diabetes, retinopathy, albuminuria without gross hematuria, and gradually progressive loss of eGFR. However, signs of DKD may be present at diagnosis [2, 43, 44] or without retinopathy in T2DM [45]. Reduced eGFR without albuminuria has been frequently reported in T2DM and is becoming more common [46]. We could have difficulties in differentiating patients with DKD and without albuminuria or reduced eGFR from patients with NDKD. The associations might have been stronger if more rigorous diagnostic methods were adopted.
Third, we applied BIA instead of standard methods such as computed tomography (CT) or dual-energy X-ray absorptiometry (DXA) [47] to measure body composition in our study. CT has limitations such as radiation risk, high cost, and not being suitable for ubiquitous and frequent use. DXA is expensive and requires specialized radiology equipment and thus is hardly feasible in routine clinical practice. However, BIA is commonly available and used in clinical practice and research studies to evaluate body composition including VFA. This method measures electrical data including resistance, reactance, and impedance of the body that are less affected by factors such as daily diet or exercise [48]. Moreover, BIA has good reliability, accuracy, and clinical feasibility when compared with standard methods in healthy and obesity people, as well as subjects with T2DM [7, 49,50,51,52]. In addition, the correlation of daily dietary intake of nutrients and exercise with the nutrition parameters estimated by BIA might be interesting, and more rigorous research is needed to understand how to design and implement these programs for populations with diabetes.
Conclusion
We identified two clusters by factor analysis, namely a body fat cluster consisting of VFA, BMI, WC, and PBF and a nutritional parameters cluster consisting of PA50, hemoglobin, BCM, and albumin, in patients with T2DM and report novel associations of poor nutritional parameters with decreased eGFR and increased UACR. Concurrently, we demonstrated that combination of high body fat with poor nutritional parameters was significantly associated with increased odds of having DKD in our cohort of participants with T2DM. Although the BIA method and consequent findings need further studies for confirmation, our results suggest that body fat and nutritional parameters could be a strong risk marker of DKD and have promise for clinical diagnosis and interventions.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997.
Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316(6):602–10.
Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905–6.
Zhang XX, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in china: a meta-analysis of observational studies. J Diabetes Res. 2020;2020:2315607.
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45.
Jiang W, Wang J, Shen X, et al. Establishment and validation of a risk prediction model for early diabetic kidney disease based on a systematic review and meta-analysis of 20 cohorts. Diabetes Care. 2020;43(4):925–33.
Pietilainen KH, Kaye S, Karmi A, Suojanen L, Rissanen A, Virtanen KA. Agreement of bioelectrical impedance with dual-energy X-ray absorptiometry and MRI to estimate changes in body fat, skeletal muscle and visceral fat during a 12-month weight loss intervention. Br J Nutr. 2013;109(10):1910–6.
Al-Sofiani ME, Ganji SS, Kalyani RR. Body composition changes in diabetes and aging. J Diabetes Complicat. 2019;33(6):451–9.
Sasongko MB, Widyaputri F, Sulistyoningrum DC, et al. Estimated resting metabolic rate and body composition measures are strongly associated with diabetic retinopathy in Indonesian adults with type 2 diabetes. Diabetes Care. 2018;41(11):2377–84.
Chen X, Mao Y, Hu J, et al. Perirenal fat thickness is significantly associated with the risk for development of chronic kidney disease in patients with diabetes. Diabetes. 2021;70(10):2322–32.
ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(Supplement 1):S19–40.
ElSayed NA, Aleppo G, Aroda VR, et al. 11. Chronic kidney disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S191–202.
Shi C, He A, Wu X, et al. Urinary IL-18 is associated with arterial stiffness in patients with type 2 diabetes. Front Endocrinol (Lausanne). 2022;13:956186.
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e426–83.
Lee AK, Katz R, Jotwani V, et al. Distinct dimensions of kidney health and risk of cardiovascular disease, heart failure, and mortality. Hypertension. 2019;74(4):872–9.
Bullen AL, Katz R, Jotwani V, et al. Biomarkers of kidney tubule health, CKD progression, and acute kidney injury in SPRINT (Systolic Blood Pressure Intervention Trial) participants. Am J Kidney Dis. 2021;78(3):361–8.
Ueshima J, Momosaki R, Shimizu A, et al. Nutritional assessment in adult patients with dysphagia: a scoping review. Nutrients. 2021;13(3):778.
Taberna DJ, Navas-Carretero S, Martinez JA. Current nutritional status assessment tools for metabolic care and clinical nutrition. Curr Opin Clin Nutr Metab Care. 2019;22(5):323–8.
Dittmar M, Reber H, Kahaly GJ. Bioimpedance phase angle indicates catabolism in type 2 diabetes. Diabet Med. 2015;32(9):1177–85.
Han BG, Lee JY, Kim JS, Yang JW. Decreased bioimpedance phase angle in patients with diabetic chronic kidney disease stage 5. Nutrients. 2019;11(12):2874.
Levi R, Bleich SN, Seligman HK. Food insecurity and diabetes: overview of intersections and potential dual solutions. Diabetes Care. 2023;46(9):1599–608.
Man RE, Sabanayagam C, Chiang PP, et al. Differential association of generalized and abdominal obesity with diabetic retinopathy in asian patients with type 2 diabetes. JAMA Ophthalmol. 2016;134(3):251–7.
Kim SR, Yoo JH, Song HC, et al. Relationship of visceral and subcutaneous adiposity with renal function in people with type 2 diabetes mellitus. Nephrol Dial Transplant. 2011;26(11):3550–5.
Hu J, Yang S, Zhang A, et al. Abdominal obesity is more closely associated with diabetic kidney disease than general obesity. Diabetes Care. 2016;39(10):e179–80.
Kahn DE, Bergman BC. Keeping it local in metabolic disease: adipose tissue paracrine signaling and insulin resistance. Diabetes. 2022;71(4):599–609.
Cypess AM. Reassessing human adipose tissue. N Engl J Med. 2022;386(8):768–79.
Fu Z, Wu Q, Guo W, et al. Impaired insulin clearance as the initial regulator of obesity-associated hyperinsulinemia: novel insight into the underlying mechanism based on serum bile acid profiles. Diabetes Care. 2022;45(2):425–35.
Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239.
Vijay J, Gauthier MF, Biswell RL, et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2(1):97–109.
Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22.
Maliszewska K, Kretowski A. Brown adipose tissue and its role in insulin and glucose homeostasis. Int J Mol Sci. 2021;22(4).
Tuttle KR, Agarwal R, Alpers CE, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022;102(2):248–60.
Tanamas SK, Saulnier PJ, Fufaa GD, et al. Long-term effect of losartan on kidney disease in American Indians with type 2 diabetes: a follow-up analysis of a randomized clinical trial. Diabetes Care. 2016;39(11):2004–10.
Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387(22):2021–32.
Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4S):S1–115.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86.
Thomas MC, Coughlan MT, Cooper ME. The postprandial actions of GLP-1 receptor agonists: the missing link for cardiovascular and kidney protection in type 2 diabetes. Cell Metab. 2023;35(2):253–73.
Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29.
Kitada M, Ogura Y, Monno I, Koya D. A low-protein diet for diabetic kidney disease: its effect and molecular mechanism, an approach from animal studies. Nutrients. 2018;10(5):544.
Ojo O. Dietary intake and type 2 diabetes. Nutrients. 2019;11(9):2177.
Bermejo S, Pascual J, Soler MJ. The current role of renal biopsy in diabetic patients. Minerva Med. 2018;109(2):116–25.
Gonzalez Suarez ML, Thomas DB, Barisoni L, Fornoni A. Diabetic nephropathy: IS it time yet for routine kidney biopsy? World J Diabetes. 2013;4(6):245–55.
Wu B, Zhang S, Lin H, Mou S. Prevention of renal failure in Chinese patients with newly diagnosed type 2 diabetes: a cost-effectiveness analysis. J Diabetes Investig. 2018;9(1):152–61.
de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–9.
He F, Xia X, Wu XF, Yu XQ, Huang FX. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia. 2013;56(3):457–66.
Oshima M, Shimizu M, Yamanouchi M, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol. 2021;17(11):740–50.
Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB. Body composition by DXA. Bone. 2017;104:101–5.
Jackson AA, Johnson M, Durkin K, Wootton S. Body composition assessment in nutrition research: value of BIA technology. Eur J Clin Nutr. 2013;67(Suppl 1):S71–8.
Huang AC, Chen YY, Chuang CL, et al. Cross-mode bioelectrical impedance analysis in a standing position for estimating fat-free mass validated against dual-energy x-ray absorptiometry. Nutr Res. 2015;35(11):982–9.
Omura-Ohata Y, Son C, Makino H, et al. Efficacy of visceral fat estimation by dual bioelectrical impedance analysis in detecting cardiovascular risk factors in patients with type 2 diabetes. Cardiovasc Diabetol. 2019;18(1):137.
Coeffier M, El Machkouri M, L’Huillier C, et al. Accuracy of bioimpedance equations for measuring body composition in a cohort of 2134 patients with obesity. Clin Nutr. 2022;41(9):2013–24.
Sun G, French CR, Martin GR, et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr. 2005;81(1):74–8.
Acknowledgements
The authors thank the investigators, staff, and trial participants for dedication and commitment to the trial.
Funding
This study received support from Natural Science Foundation of Jiangsu province: General program BK20201497 Yang Zhou, and National Natural Science Foundation of China: General program 82270760 to Junwei Yang. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Yang Zhou and Junwei Yang contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Aiqin He, Caifeng Shi, Xiaomei Wu, Yuting Sheng and Xueting Zhu. The first draft of the manuscript was written by Yang Zhou. Junwei Yang, Aiqin He, Caifeng Shi, and Xiaomei Wu commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Aiqin He, Caifeng Shi, Xiaomei Wu, Yuting Sheng, Xueting Zhu, Junwei Yang, and Yang Zhou have nothing to declare.
Ethics Approval
This study adhered to the principles of the Declaration of Helsinki and the protocols were approved by the ethics committee of Nanjing Medical University (Approval No. 2019KY097). The ethics clearance, information sheet, and consent form were approved by the faculty institutional review board. Study participants were outpatients visiting the department of Nephrology and Endocrinology, Second Affiliated Hospital of Nanjing Medical University. We obtained written informed consent from all participants. No identifying information was included in the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
He, A., Shi, C., Wu, X. et al. Clusters of Body Fat and Nutritional Parameters are Strongly Associated with Diabetic Kidney Disease in Adults with Type 2 Diabetes. Diabetes Ther 15, 201–214 (2024). https://doi.org/10.1007/s13300-023-01502-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01502-5