Abstract
Introduction
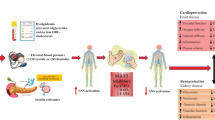
Hyperinsulinemia and hyperglycemia are associated with exaggerated systemic sympathetic nerve activity (SNA) in patients with type 2 diabetes. Sodium–glucose cotransporter 2 (SGLT2) inhibitors lower insulin levels, whereas sulfonylureas increase insulin levels. We will test whether these two classes of antidiabetic agents have different effects on SNA.
Methods
The present study is an ongoing, 24-week, one-center (only Kanazawa University Hospital), open-label, randomized, parallel trial (jRCTs 041200035). Participants with type 2 diabetes with multiple atherosclerosis risk factors are randomly assigned in a 1:1 manner to receive 2.5 mg luseogliflozin or 0.5 mg glimepiride once daily. The sample size was calculated to be 14 in each group, with a significance level of 0.05 and a power of 0.80. The design required 40 evaluable study participants. Our primary endpoint will be the change in muscle SNA (MSNA). The secondary endpoints included organ-specific insulin sensitivity measured by a hyperinsulinemic-euglycemic clamp study using an artificial pancreas combined with a stable isotope-labeled glucose infusion, bioelectrical impedance analysis, and organ-specific (cardiac, renal, and hepatic) 123I-meta-iodobenzylguanidine (MIBG) innervation imaging.
Planned Outcomes
Study recruitment started in April 2020 and will end in June 2024, with 40 participants randomized into the two groups. The treatment follow-up of the participants is currently ongoing and is due to finish by March 2025.
Trial Registration
The study protocol has been approved by the Certified Review Board, Kanazawa University, Ishikawa, Japan, in accordance with the guidelines stipulated in the Declaration of Helsinki (CRB4180005, 2019-001). This trial is registered with the Japan Registry of Clinical Trials, jRCTs 041200035.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Exaggerated sympathetic nerve activity (SNA) is associated with worsening prognosis in patients. A past single-arm trial lacks a comparative group, which precludes meaningful conclusions since glycemic control may affect autonomic nervous activity. |
Given that hyperglycemia and obesity/hyperinsulinemia activate SNA, discriminating the effects of glucose and insulin on SNA in the SGLT2 inhibitor-mediated SNA reduction is necessary. We aim to compare the effects between SGLT2 inhibitors and sulfonylureas, both of which lower glucose levels with elevation and reduction in circulating levels of insulin, respectively, in participants with type 2 diabetes. |
Using many metabolic markers, we may be able to clarify the mechanisms underlying the SGLT2 inhibitor/sulfonylurea-mediated alteration in organ-specific SNAs and insulin sensitivities. |
Introduction
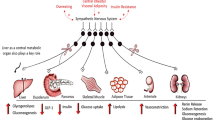
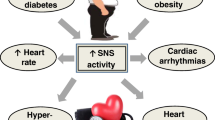
Exaggerated sympathetic nerve activity (SNA) is associated with worsening prognosis in patients with heart failure, type 2 diabetes, and cardiovascular diseases (CVD) [1, 2]. Human studies have clarified the role of the sympathetic nervous system in the pathophysiology of end-organ damage and the occurrence of major clinical complications [3]. Experimental animal models and studies in humans have conclusively shown that sympathetic neural factors have a powerful excitatory influence on cardiovascular structures, such as the heart and arteries [3]. Patients with type 2 diabetes mellitus are at risk for developing atherosclerotic CVD [4]. Hyperinsulinemia, hyperglycemia, and hypoglycemia, which occur during diabetic treatment, lead to exaggerated SNA [5, 6]. Therefore, antidiabetic treatments that do not overstimulate the SNA are expected to prevent cardiovascular complications.
In addition to hypoglycemic attacks, some diabetic agents modulate SNA. Metformin [6], pioglitazone [7], and basal insulin glargine therapy [8] are reported to reduce cardiac autonomic nervous activity, assessed by heart rate variability. Sulfonylureas remain reliable and potent antidiabetic agents in insulinopenic patients with type 2 diabetes and are, therefore, used as second-line therapies, especially when the cost is a major issue. Despite their advantage in lowering glucose levels, sulfonylureas elevate insulin levels and weight [9], which may positively and negatively affect the SNA.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors reportedly reduce CVD, heart failure, and all-cause mortality risk in patients with type 2 diabetes [10,11,12,13,14]. In a streptozotocin-diabetic rat model, SGLT2 inhibitor lowered SNA and improved baroreflex sensitivity (BRS) [15]. In humans, muscle SNA (MSNA) recordings are the gold standard for directly measuring sympathetic nervous outflow [16]. We previously demonstrated in a single-arm trial that dapagliflozin decreases MSNA in participants with type 2 diabetes and heart failure [17].
SGLT2 inhibitors were non-inferior to sulfonylureas in reducing HbA1c at 52 weeks in a phase 3 trial [18] and past report [19]. However, the differences between SGLT2 inhibitors and sulfonylureas in their effects on SNA in participants with type 2 diabetes under similar glucose level reduction remain uncertain. Given that hyperglycemia and obesity/hyperinsulinemia activate SNA, discriminating the effects of glucose and insulin on SNA in the SGLT2 inhibitor-mediated SNA reduction is necessary. For this purpose, we aim to compare the effects between SGLT2 inhibitors and sulfonylureas (active control), both of which lower glucose levels with elevation and reduction in circulating levels of insulin, respectively, in participants with type 2 diabetes.
In the present study, we aim to investigate the efficacy of the SGLT2 inhibitor luseogliflozin and sulfonylurea glimepiride on MSNA in participants with type 2 diabetes and evaluate whether the responses are related to BRS for 24 weeks in an open-label, randomized, parallel study. Through this study, we will establish the techniques for evaluating organ-specific SNA.
Methods
Study Design
This study is a 24-week, one-center, open-label, randomized, parallel trial on treatment with 2.5 mg luseogliflozin or 0.5 mg glimepiride for adults with type 2 diabetes. The study protocol is approved by the Certified Review Board, Kanazawa University, Ishikawa, Japan, in accordance with the guidelines stipulated in the Declaration of Helsinki (CRB4180005, 2019-001). This trial is registered with the Japan Registry of Clinical Trials, jRCTs 041200035. Written informed consent is obtained from all patients before study enrollment. Study recruitment started in April 2020 and will end in June 2024, with 40 participants randomized into the two groups. The treatment follow-up of the participants is currently ongoing and is due to finish by March 2025.
Sample Selection
Eligibility for the Trial
Eligible adults (age ≥ 20 years) are identified and recruited at Kanazawa University Hospital from April 2020 until June 2024. All participants provide informed written consent at the beginning of the screening visit before inclusion in this study. Specifically, we carefully explain the current clinical recommendations [20, 21] and obtain full informed consent to assign participants with multiple atherosclerosis risk factors to receive either of the agents. These risk factors are previous history of CVDs, ischemic stroke, and heart failure and/or at least one of the following conditions [hypertension, dyslipidemia, older age (male, > 50 years; female, > 55 years), smoking history, and obesity (body mass index > 25 kg/m2)].
Eligibility for the trial is determined at screening using standard blood tests, clinical history taking (including written confirmation of drug history, where necessary), and physical examination/observations to identify other illnesses or contraindications. The participants satisfying the eligibility criteria for the 24-week treatment trial are given the opportunity to participate in metabolic mechanistic sub-studies, including the hyperinsulinemic-euglycemic clamp study with stable isotope-labeled glucose infusion at visits 1 and 2. A patient’s decision to participate in or withdraw from the metabolic sub-study did not affect their participation in the main 24-week trial.
The trial entry criteria are based on type 2 diabetes within 4 weeks of screening. All participants had to be aged ≥ 20 years during the initial screening. Participants with type 2 diabetes during screening should have glycemic control (HbA1c of ≥ 7%) and have been managed by either diet and/or a stable dose of hypoglycemic agents for at least 4 weeks.
The exclusion criteria are as follows: (1) luseogliflozin or glimepiride hypersensitivity or contraindications, (2) treatment with glinide, sulfonylurea, and SGLT2 inhibitor including luseogliflozin within 4 weeks of screening, (3) type 1 diabetes or gestational diabetes, (4) diabetic ketoacidosis, hyperosmolar hyperglycemic state, and poorly controlled unstable diabetes (ketoacidosis or HbA1c increase of > 3% in the 12 weeks before screening), (5) absolute indications for insulin therapy (severely infected states, scheduled operation, severe trauma, and so on), (6) history of hypoglycemic symptoms, (7) poorly controlled hypertension or systolic or diastolic BPs (blood pressures) of > 160 or > 100 mmHg, respectively, (8) severe retinopathy requiring immediate treatment, (9) receiving treatment for malignancy, (10) severe health problem not suitable for study inclusion, (11) pregnant or breastfeeding women, and (12) inability to participate in the study (including psychiatric and psychosocial problems), as assessed by the investigators.
Randomization
The participants meeting all eligibility criteria and provided written informed consent are randomly assigned on a 1:1 basis to either of the two study treatments using computer-generated randomization at Kanazawa University Hospital.
The SGLT2 inhibitor group received luseogliflozin (starting from 2.5 mg/day and titrated up to 5.0 mg/day; brand name Lusefi, Taisho pharmaceutical holdings, Japan), whereas the sulfonylurea group received glimepiride (starting from 0.5 mg/day and titrated up to 6.0 mg/day; Sanofi K.K., Canada) for 24 weeks.
Study Intervention
The study is divided into the following four stages (Fig. 1): (1) screening, enrollment, randomization, (2) baseline investigations (visits 1 and 2, over a maximum period of 7 days), (3) study treatment (visit 3 at 12 weeks), (4) endpoint assessment at 24 weeks, including MSNA and metabolic sub-studies (visits 4 and 5, over a maximum period of 7 days). If the trial investigator or participant suspected an adverse event, an unscheduled visit is arranged within 7 days. The study visits and data collection schedules are summarized in Table 1. All participants are asked to attend each visit under fasting states for at least 9 h before each visit. A follow-up MSNA is obtained after the completion of the 24-week study treatment.
Previous treatment with oral antidiabetic drugs is continued at the same dose in participants at randomization. Glycemic control is assessed at each 12-week trial visit using plasma glucose and HbA1c measurements. In the event of glycemic control deterioration defined as HbA1c of > 9% even if the allocated drug is dosed up after the intervention, the participant will be informed and counseled about starting the open-label, long-acting insulin analogues once daily. However, the patient’s trial participation will not be jeopardized if they do not wish to take the long-acting insulin analogues. The dose of the long-acting insulin analogues is titrated by trial investigators to ensure that the standard of diabetes care will not be significantly compromised as a result of the patient’s participation in our clinical trial. In addition to the study medications, the participants continued to undergo lifestyle modifications (i.e., exercise, weight loss, and dietary adjustment), and their coexisting illnesses are managed throughout the trial. The participants are not allowed to take any new prescriptions.
Planned Outcomes
The primary outcome measure will be assessed using an intention-to-treat analysis of the MSNA changes, i.e., burst frequency (BF, bursts/minute) and burst incidence (BI, bursts/100 heartbeats), from the beginning to 24 weeks of treatment.
Secondary endpoints included changes in (1) BF and BI from the beginning to 12 weeks of treatment, (2) BRS by MSNA and CVR-R interval, (3) cardiac autonomic nerve activity, (4) organ-specific insulin sensitivity, estimated by the hyperinsulinemic-euglycemic clamp study using an artificial pancreas, (5) oxidative and non-oxidative glucose metabolism, calculated by indirect calorimetry, (6) basal metabolism and respiration quotient, calculated by indirect calorimetry, (7) heart rate and BP, (8) body compositions, (9) blood glucose and insulin sensitivity (fasting plasma glucose levels, hemoglobin A1c, glycated albumin, immunoreactive insulin, homeostasis model assessment of insulin resistance, quantitative insulin sensitivity check index, C-peptide immunoreactivity), (10) lipid metabolism, (11) renal function and electrolyte balances, (12) adrenal hormone [plasma renin activity, plasma aldosterone levels, plasma catecholamine (adrenaline, noradrenaline, and metanephrines) levels, urinary epinephrine, norepinephrine, metanephrine, and normetanephrine], (13) oxidative stress (Fe, ferritin) levels, (14) cytokine concentration (leptin, adiponectin), (15) hepatokines (selenoprotein P and LECT-2) levels, (16) evaluation of safety, (17) iodine-123 meta-iodobenzylguanidine (123I-MIBG) scintigraphic indices of the heart, kidney, and liver, namely, washout rate, and heart-to-mediastinum (H/M), kidney-to-background (K/BG), and liver-to-mediastinum (L/M) ratios, (18) left ventricular ejection fraction, diastolic capacity, and cardiac load measured by echocardiography, (19) endothelial functions (EndoPAT), (20) relation between organ-specific SNA and organ-specific insulin sensitivity, and (21) gene expression related to glucose and lipid metabolism.
Data Collection
MSNA
All experiments are performed in a quiet, electrically shielded room in the afternoon with the participant in the supine position. HR is determined from a continuous ECG. The beat-to-beat BP is recorded continuously from the radial artery using a non-invasive tonometry monitoring system (JENTOW-7700; Nihon Colin, Komaki, Japan). Postganglionic MSNA is recorded from the left peroneal nerve, as described previously [22,23,24]. Briefly, with the participant in a comfortable supine position, the common peroneal nerve is located by palpation and stimulated electrically via the skin surface. A tungsten microelectrode is inserted percutaneously into a motor fascicle of the peroneal nerve. The electrode is adjusted until spontaneous pulse-synchronous sympathetic burst activities could be recorded. The signal is fed through a band-pass filter (500–3000 kHz) and a resistance–capacitance integrated circuit with a time constant of 0.1 s to produce a mean voltage neurogram using the PowerLab recording system (Model ML 785/85P; ADInstruments, Bella Vista, Australia). The raw nerve signal is obtained at 12,000 Hz, whereas the other signals are obtained at 1000 Hz. In a blinded manner, multi-unit MSNA is identified offline on the basis of its relationship to cardiac activity in the integrated nerve recording by an experienced investigator. Burst activity is identified as a > 3 signal-to-noise ratio on mean voltage neurograms. For each participant, multi-unit MSNA is expressed as the number per minute (BF) (bursts/minute) and number per 100 heartbeats (BI) (bursts/100 heartbeats). Data are acquired for at least 10 min. Multi-unit MSNA has high reproducibility [24, 25].
BRS
BRS analysis spontaneous arterial baroreflex modulation of HR (cardiovagal BRS) is estimated using the sequence method [26]. The beat-to-beat time series of systolic BP (SBP) and RR interval are analyzed using HemoLab software (Harald Stauss Scientific, Iowa City, IA), as reported previously [17]. Arterial baroreflex modulation of MSNA (sympathetic BRS) is assessed by examining the relationship between MSNA occurrence and diastolic BP [17].
Holter Electrocardiogram
The participants underwent ambulatory Holter electrocardiogram monitoring. The results are transformed into frequency-domain waveforms that included high-frequency (HF) (0.15–0.40 Hz) and low-frequency (LF) (0.04–0.15 Hz) powers. LF power, HF power, and LF/HF ratio represent SNA, parasympathetic nerve activity, and sympathovagal balance, respectively [27].
Clinical Information
Clinical information, including age, sex, medication, and body measurements, is obtained for each patient. Body measurements, including height, weight, heart rate, systolic/diastolic BP, and waist circumferences, are recorded. Body composition, including body fat mass and fat-free mass, is determined by a bioelectrical impedance analysis (Tanita BC-118D; Tanita, Tokyo, Japan), and a suitable commercial octapolar BIA system (InBody 720, InBody Co., Ltd., Tokyo, Japan). Venous blood samples obtained after overnight fasting are used to evaluate blood chemistry, including full blood count, liver enzymes (serum aspartate aminotransferase, serum alanine aminotransferase, and plasma gamma-glutamyltransferase), renal function (blood urea nitrogen, creatinine, and estimated glomerular filtration rate), electrolyte levels, lipid profile (total cholesterol, high-density lipoprotein, and triglycerides), HbA1c, and plasma glucose.
Organ-Specific Insulin Sensitivity
Organ-specific insulin sensitivity is estimated via a hyperinsulinemic-euglycemic clamp study using an artificial pancreas (model STG-55; Nikkiso, Tokyo, Japan) combined with a stable isotope-labeled [6,6-2H2]glucose infusion. We calculated and defined organ-specific insulin resistance in the liver, skeletal muscle, and adipose tissue, as described previously [28]. Hepatic insulin resistance indices are calculated as the product of fasting hepatic glucose production (HGP) and fasting plasma insulin concentration and HGP suppression by insulin during a clamp study. The skeletal muscle insulin resistance index is calculated as insulin-stimulated glucose disposal (Rd) [29, 30].
Energy Metabolism
Whole-body indirect calorimetry is performed during the hyperinsulinemic-euglycemic clamp study using an AE-310 s Aeromonitor (Minato Medical Science Company, Osaka, Japan). Respiratory quotient is defined as the ratio of carbon dioxide production (VCO2) to the whole-body oxygen consumption (VO2). Basal energy expenditure is calculated from VO2 and VCO2 using the Weir equation. We calculated glucose oxidation from the respiratory gas exchange and nitrogen excretion in the urine [31]. Non-oxidative glucose disposal is estimated by subtracting the glucose oxidation rate from Rd [32].
123I-MIBG Images
123I-MIBG images are obtained using a double-headed gamma camera interfaced with a dedicated nuclear medicine workstation (Siemens Healthcare, Tokyo, Japan). The camera is equipped with a low-to-medium-energy general-purpose collimator. The energy window is centered on 159 keV with a 15% window. A planar anterior image that included the heart and liver and a planar posterior image that included the liver and kidneys are acquired for 3 min at 20 min (early) and 3 h (delayed) after injection of 111 MBq of 123I-MIBG (FUJIFILM Toyama Chemical Co. Ltd, Tokyo, Japan). The image matrix is 256 × 256 pixels. For quantitative analysis, circular or elliptical regions of interest (ROIs) are positioned over the heart (H), liver (L), and kidney (K) images. A rectangular ROI is placed over the upper one-third of the mediastinum (M) and background (BG) in the anterior and posterior images, respectively. The ROIs of H and M are according to the published 123I-MIBG imaging guidelines [33]. An elliptical ROI is set on a kidney, and a rectangular BG ROI is set between the kidneys. For calculating the H/M, L/M, and K/BG ratios, the ROIs are analyzed with a Mathematica (Wolfram Research Inc., Champaign, IL. USA) program created for this purpose. The radiotracer washout rate (WR) is calculated as (early H count − delayed H count/k)/early H × 100(%), where k is a decay correction factor calculated as 0.5^(time[h]/13). An experienced nuclear medicine physician blinded to patient information performed the quantitative image analysis. K/BG ratios and WR values (%) are obtained for a kidney that did not include excreted renal retention activity and liver activity overlap. The reproducibility of the indices by 123I-MIBG imaging quantitation is assessed in advance.
Echocardiography
Echocardiography (Aplio; Toshiba, Tokyo, Japan) is performed on the same day or within a week before the MSNA measurement. Left ventricular ejection fraction is measured by using the Simpson method. E/e′ is calculated by using the E wave derived from the transmittal flow per e′ measured by tissue Doppler imaging of the left ventricular basal septal wall.
Peripheral endothelial function is measured using an EndoPAT-2000 device (Itamar Medical, Caesarea, Israel). A peripheral arterial tonometry probe is attached to the fingers of both hands of the participant. The reactive hyperemia index (RHI) is determined according to the manufacturer’s instructions, with RHI < 1.67 indicating endothelial dysfunction [34].
The participants’ peripheral whole blood is collected periodically, placed in PAXgene Blood RNA Tubes (PreAnalytiX GmbH, Hombrechtikon, Switzerland) to stabilize the RNA, and stored per the manufacturer’s protocol.
Case Report Forms (CRFs)
The CRFs included baseline/follow-up medical histories and physical examinations to capture comorbidities and concomitant medications in the trial’s database. Other CRFs incorporated in the database included the following: laboratory data, including questionnaire results; safety monitoring during the follow-up periods; MSNA reports; 123I-MIBG imaging; organ-specific insulin sensitivity; and adverse-event reporting and study-drug dispensing forms for study treatment adherence and accountability. Treatment compliance, including the evidence of dosage and clinical events, will be monitored at each study visit.
Data Analysis
Sample Size Estimation
The present study is powered to detect a change in the primary endpoint, MSNA BF. A priori sample size calculations assumed a 15–20% reduction in MSNA according to our previous studies [17, 35]. We calculated the sample size as 12 in each group, with a significance level of 0.05 (type I error) and a power of 0.80 (type II error). Published clinical trials reported an average participant withdrawal rate of 10–20%. This design required 40 evaluable participants in the treatment group. Therefore, we randomize our recruitment target of 40 participants in a 1:1 allocation ratio to either luseogliflozin or glimepiride.
Statistical Analysis
Statistical analyses will be performed with an intention-to-treat principle to ascertain safety and adverse effects. The baseline characteristics of the two study groups are summarized with frequencies for categorical variables and means (standard deviation) for normally distributed variables or median (interquartile range) for non-normally distributed continuous variables. We will perform the Shapiro–Wilk test to evaluate the assumption of a normal distribution. The between-group comparison at baseline will be performed with the χ2 test or Fisher’s exact test for categorical variables and Mann–Whitney U test for non-normally distributed data or two-sample t test for normally distributed data for continuous parameters. The intergroup comparison of the continuous parameters will be performed with the Mann–Whitney U test for the non-normally distributed data or the two-sample t test for the normally distributed data. The internal group comparison at baseline and 24 weeks will be performed with the Wilcoxon-signed rank test. Data pertaining to the major clinical events of interest are presented as frequencies and percentages for categorical variables. We will examine the association between the baseline characteristics and laboratory data changes by performing a Spearman analysis. P < 0.05 will be regarded as statistically significant. All analyses will be performed with SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY).
We state that similar methodologies have been presented previously [36].
Discussion
This study will be the first to report on the efficacy of treatment with the SGLT2 inhibitor and sulfonylurea on organ-specific SNA in participants with type 2 diabetes in a 24-week open-label randomized trial. The enrollment of the required sample size will be completed in June 2024, and the final results are expected by the end of 2025.
Diabetic treatment dynamically alters cardiac autonomic nervous activity [6,7,8, 17]. We found that insulin glargine elevates the parasympathetic tone at nighttime and reduces the SNA at dawn [8]. Hence, insulin glargine might prevent cardiovascular events by reducing the sympathetic tone at dawn. Additionally, dapagliflozin in a single-arm study decreases MSNA [17]. However, these studies lack a comparative group, which precludes meaningful conclusions since glycemic control may affect the autonomic nervous activity. Our study will compare the effects between the SGLT2 inhibitor and sulfonylurea on MSNA in participants with type 2 diabetes. Through this study, we will elucidate the pleiotropic effects of the SGLT2 inhibitor on organ-specific SNAs and insulin sensitivity by comparing them with those of the active control sulfonylurea, which elevates insulin secretion and with similar glucose reduction to that of the SGLT2 inhibitor.
In addition to the primary endpoint MSNA, we will evaluate the organ-specific SNA as the secondary endpoint by 123I-MIBG imaging. The 123I-MIBG scintigraphy is a noninvasive imaging method for assessing SNA. We found that the renal 123I-MIBG washout rates are correlated significantly with the gold standard SNA indicator MSNA [35]. We previously reported that the SGLT2 inhibitor reduces MSNA [17]. However, it remains unclear whether the SGLT2 inhibitor directly or indirectly affects the renal 123I-MIBG washout rates. The afferent SNA in the kidney is stimulated as the efferent SNA increases [37], which leads to deterioration of CVDs such as hypertension. A recent large-scale randomized trial showed that SGLT2 inhibitors preserved kidney functions in participants with chronic kidney disease (DAPA-CKD) [38]. However, no human study has demonstrated the sympathetic response to antidiabetic drugs in the kidneys and other organs. Our present study will clarify the organ-specific SNAs and their differences among the diabetic agents. The assessment of renal SNA will deepen our understanding of the pleiotropic actions of antidiabetic agents and contribute to tailored antidiabetic medicine in the future.
The secondary endpoints in the present study include organ-specific insulin sensitivity evaluated by the hyperinsulinemic-euglycemic clamp study combined with stable isotope-labeled glucose infusion. The process on how SNA modulates insulin signaling remains unclear. The SGLT2 inhibitor reduces body weight and hepatic fat accumulation and inflammation [19], which are often recognized to be associated with insulin resistance. However, liver fat is associated with skeletal muscle insulin resistance, but not with insulin resistance in the liver and adipose tissue [29]. In contrast, hepatic glycogen deficiency enhances lipolysis in the adipose tissue to generate glycerol that fuels hepatic gluconeogenesis and thereby reduces liver fat via a liver–brain–adipose neurocircuitry. These interorgan networks should be considered when interpreting our findings in organ-specific SNAs and insulin sensitivities.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Thaung HPA, Baldi JC, Wang H-Y, et al. Increased efferent cardiac sympathetic nerve activity and defective intrinsic heart rate regulation in type 2 diabetes. Diabetes. 2015;64(8):2944–56. https://doi.org/10.2337/db14-0955.
Minatoguchi S. Heart failure and its treatment from the perspective of sympathetic nerve activity. J Cardiol. 2022;79(6):691–7. https://doi.org/10.1016/j.jjcc.2021.11.016.
Grassi G, Seravalle G, Dell’Oro R, Mancia G. Sympathetic mechanisms, organ damage, and antihypertensive treatment. Curr Hypertens Rep. 2011;13(4):303–8. https://doi.org/10.1007/s11906-011-0200-4.
Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41. https://doi.org/10.1056/NEJMoa1008862.
Limberg JK, Smith JA, Soares RN, et al. Sympathetically mediated increases in cardiac output, not restraint of peripheral vasodilation, contribute to blood pressure maintenance during hyperinsulinemia. Am J Physiol Circ Physiol. 2020;319(1):H162-70. https://doi.org/10.1152/ajpheart.00250.2020.
Manzella D. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: effect of metformin administration. Am J Hypertens. 2004;17(3):223–7. https://doi.org/10.1016/j.amjhyper.2003.11.006.
Kobayashi D, Takamura M, Murai H, et al. Effect of pioglitazone on muscle sympathetic nerve activity in type 2 diabetes mellitus with alpha-glucosidase inhibitor. Auton Neurosci Basic Clin. 2010;158(1–2):86–91. https://doi.org/10.1016/j.autneu.2010.05.003.
Takeshita Y, Tanaka T, Wakakuri H, Kita Y, Kanamori T, Takamura T. Metabolic and sympathovagal effects of bolus insulin glulisine versus basal insulin glargine therapy in people with type 2 diabetes: a randomized controlled study. J Diabetes Investig. 2021;12(7):1193–201. https://doi.org/10.1111/jdi.13471.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720.
Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs. Circulation. 2017;136(3):249–59. https://doi.org/10.1161/CIRCULATIONAHA.117.029190.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. https://doi.org/10.1056/NEJMoa1611925.
Norhammar A, Bodegård J, Nyström T, Thuresson M, Nathanson D, Eriksson JW. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE-TIMI 58 trial: a nationwide observational study. Diabetes, Obes Metab. 2019;21(5):1136–45. https://doi.org/10.1111/dom.13627.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9. https://doi.org/10.1016/S0140-6736(18)32590-X.
Yoshikawa T, Kishi T, Shinohara K, et al. Arterial pressure lability is improved by sodium-glucose cotransporter 2 inhibitor in streptozotocin-induced diabetic rats. Hypertens Res. 2017;40(7):646–51. https://doi.org/10.1038/hr.2017.14.
Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59(4):919–57. https://doi.org/10.1152/physrev.1979.59.4.919.
Hamaoka T, Murai H, Hirai T, et al. Different responses of muscle sympathetic nerve activity to dapagliflozin between patients with type 2 diabetes with and without heart failure. J Am Heart Assoc. 2021. https://doi.org/10.1161/JAHA.121.022637.
Cefalu WT, Leiter LA, Yoon K-H, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–50. https://doi.org/10.1016/S0140-6736(13)60683-2.
Takeshita Y, Honda M, Harada K, et al. Comparison of tofogliflozin and glimepiride effects on nonalcoholic fatty liver disease in participants with type 2 diabetes: a randomized, 48-week, open-label active-controlled trial. Diabetes Care. 2022;45(9):2064–75. https://doi.org/10.2337/dc21-2049.
ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2023. Diabetes Care. 2023;46(Supplement_1):140–57. https://doi.org/10.2337/dc23-S009.
Bouchi R, Kondo T, Ohta Y, et al. A consensus statement from the Japan Diabetes Society: a proposed algorithm for pharmacotherapy in people with type 2 diabetes. J Diabetes Investig. 2023;14(1):151–64. https://doi.org/10.1111/jdi.13960.
Murai H, Takamura M, Maruyama M, et al. Altered firing pattern of single-unit muscle sympathetic nerve activity during handgrip exercise in chronic heart failure. J Physiol. 2009;587(11):2613–22. https://doi.org/10.1113/jphysiol.2009.172627.
Inomata J, Murai H, Kaneko S, et al. Differential effects of azelnidipine and amlodipine on sympathetic nerve activity in patients with primary hypertension. J Hypertens. 2014;32(9):1898–904. https://doi.org/10.1097/HJH.0000000000000270.
Hamaoka T, Murai H, Kaneko S, et al. Single-unit muscle sympathetic nerve activity reflects sleep apnea severity, especially in severe obstructive sleep apnea patients. Front Physiol. 2016. https://doi.org/10.3389/fphys.2016.00066.
Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3(3):201–5. https://doi.org/10.1007/BF01826234.
Parati G, Di Rienzo M, Bertinieri G, et al. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension. 1988;12(2):214–22. https://doi.org/10.1161/01.HYP.12.2.214.
Perini R, Veicsteinas A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol. 2003;90(3–4):317–25. https://doi.org/10.1007/s00421-003-0953-9.
Cobelli C, Mari A, Ferrannini E. Non-steady state: error analysis of Steele’s model and developments for glucose kinetics. Am J Physiol. 1987;252(5 Pt 1):E679-89. https://doi.org/10.1152/ajpendo.1987.252.5.E679.
Kato K, Takamura T, Takeshita Y, et al. Ectopic fat accumulation and distant organ-specific insulin resistance in Japanese people with nonalcoholic fatty liver disease. PLoS ONE. 2014;9(3):e92170. https://doi.org/10.1371/journal.pone.0092170.
Takeshita Y, Teramura C, Kamoshita K, et al. Effects of eicosapentaenoic acid on serum levels of selenoprotein P and organ-specific insulin sensitivity in humans with dyslipidemia and type 2 diabetes. J Diabetes Investig. 2022;13(3):532–42. https://doi.org/10.1111/jdi.13699.
Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–34. https://doi.org/10.1152/jappl.1983.55.2.628.
Yokoyama H, Mori K, Emoto M, et al. Non-oxidative glucose disposal is reduced in type 2 diabetes, but can be restored by aerobic exercise. Diabetes, Obes Metab. 2008;10(5):400–7. https://doi.org/10.1111/j.1463-1326.2007.00716.x.
Flotats A, Carrió I, Agostini D, et al. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging. 2010;37(9):1802–12. https://doi.org/10.1007/s00259-010-1491-4.
Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–41. https://doi.org/10.1016/j.jacc.2004.08.062.
Takamura M, Murai H, Okabe Y, et al. Significant correlation between renal 123I-metaiodobenzylguanidine scintigraphy and muscle sympathetic nerve activity in patients with primary hypertension. J Nucl Cardiol. 2017;24(2):363–71. https://doi.org/10.1007/s12350-016-0760-4.
Takeshita Y, Kanamori T, Tanaka T, et al. study protocol for pleiotropic effects and safety of sodium-glucose cotransporter 2 inhibitor versus sulfonylurea in patients with type 2 diabetes and nonalcoholic fatty liver disease. Diabetes Ther. 2020. https://doi.org/10.1007/s13300-020-00762-9.
Katsurada K, Shinohara K, Aoki J, Nanto S, Kario K. Renal denervation: basic and clinical evidence. Hypertens Res. 2022;45(2):198–209. https://doi.org/10.1038/s41440-021-00827-7.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. https://doi.org/10.1056/NEJMoa2024816.
Medical Writing/Editorial Assistance
We received assistance from Enago™ English Editing Service (Crimson Interactive Pvt. Ltd.), which was paid from research Grants from Japan Association for Diabetes Education and Care (Yumie Takeshita, 2022-FND-014).
Funding
This work is supported by research Grants from Japan Association for Diabetes Education and Care (Yumie Takeshita, 2022-FND-014) and by research grants from Taisho Pharma Co., Ltd. (Toshinari Takamura). The journal’s Rapid Service fee is paid from research Grants from Japan Association for Diabetes Education and Care (Yumie Takeshita, 2022-FND-014).
Author information
Authors and Affiliations
Contributions
Yumie Takeshita: designed and conducted the study, recruited the participants, analyzed the data, and wrote the manuscript. Chiaki Nomura: recruited the participants; collected the clinical information, performed the hyperinsulinemic-euglycemic clamp study, and analyzed MSNA. Yusuke Mukai, Takuto Hamaoka, Tadayuki Hirai, Soichiro Usai, and Masayuki Takamura: performed and analyzed MSNA. Shota Tokuno, Toshinari Takamura, Hisanori Goto, and Yujiro Nakano: recruited the participants, collected the clinical information, and performed the hyperinsulinemic-euglycemic clamp study. Kenichi Nakajima: performed and analyzed123 I-MIBG images. Takeo Tanaka: designed the study, recruited the participants, interpreted the data, and edited the manuscript. All authors met the International Committee of Medical Journal Editors criteria for authorship for this work, and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
Yumie Takeshita and Toshinari Takamura have received consulting fees from Kowa Company Ltd. and Sumitomo Pharma Receipt of grants or research support: Yumie Takeshita has received lecture fees from Sumitomo Pharma, Kowa Company Ltd., and Sanofi. Toshinari Takamura has received lecture fees from MSD, Sumitomo Dainippon Pharma, Kowa Company Ltd., and Mitsubishi Tanabe Pharma Corp. Toshinari Takamura has received research grants from Taisyo PharmaCo., Kowa Company Ltd., Life Scan Japan, Sanwa Kagaku Kenkyusho Co. Ltd., SumitomoPharma, Kowa Company Ltd., Mitsubishi Tanabe Pharma Corp., Abbott, and Nippon Boehringer Ingelheim Co., Ltd. Chiaki Nomura, Hisayoshi Murai, Yusuke Mukai, Tadayuki Hirai, Takuto Hamaoka, Shota Tokuno, Takeo Tanaka, Hisanori Goto, Yujiro Nakano, Soichiro Usui, Kenichi Nakajima, and Masayuki Takamura have nothing to disclose.
Ethical Approval
The institutional review board of each participating institution has approved the study protocol in compliance with the Declaration of Helsinki and current legal regulations in Japan. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients that are willing to participate in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Takeshita, Y., Nomura, C., Murai, H. et al. Study Protocol for the Pleiotropic Effects of Sodium–Glucose Cotransporter 2 Inhibitor on Organ-Specific Sympathetic Nerve Activity and Insulin Sensitivity in Participants with Type 2 Diabetes. Diabetes Ther 15, 269–280 (2024). https://doi.org/10.1007/s13300-023-01497-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01497-z