Abstract
Introduction
Fixed ratio combination of insulin degludec and liraglutide (IDegLira) represents an option to revise inappropriate therapies in patients with poorly controlled type 2 diabetes. This study aimed to assess the pattern of use and 1-year effectiveness of IDegLira.
Methods
A retrospective chart review was performed to assess changes in glycated hemoglobin (HbA1c) (primary endpoint), fasting blood glucose (FBG), body weight, estimated glomerular filtration rate (eGFR), and lipid profile following IDegLira initiation. Previous versus concomitant diabetes treatments were also compared.
Results
Overall, 87 patients (mean age 73.9 ± 9.2 years, diabetes duration 18.2 ± 6.7 years, 62.1% men, HbA1c 8.3 ± 1.3%, BMI 30.4 ± 5.5 kg/m2) initiated IDegLira. Previously, 21.8% of patients were treated with oral hypoglycemic agents (OHA group), 47.1% with basal insulin ± OHA (BOT group), 5.8% with GLP-1 RA ± basal insulin (GLP1-RA group), and 25.3% with basal-bolus schemes (BB group). At the first prescription of IDegLira, secretagogues and schemes including two or more OHA were substantially reduced, leaving metformin as the most prevalent OHA (81.6%) used in combination with IDegLira. Starting dose of IDegLira ranged from 18.7 ± 3.1 U (OHA group) to 24.1 ± 4.4 U (BB group). After 1 year, HbA1c was significantly reduced by 1.25% (95% CI − 1.48; − 1.03), FBG by 52.9 mg/dl, and body weight by 2.0 kg. Also, eGFR levels and lipid profile significantly improved. No severe hypoglycemia occurred.
Conclusion
It is possible to proactively review suboptimal or inappropriate diabetes treatment according to the most recent guidelines. Results suggest that initiation of IDegLira was associated with a reduction in drugs to be administered daily and relevant improvements in clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Many ongoing type 2 diabetes therapies should be proactively revised to improve appropriateness and outcomes of care. |
Fixed ratio combination of insulin degludec and liraglutide (IDegLira) represents an option to revise inappropriate therapies in patients with poorly controlled type 2 diabetes. |
Pattern of use and 1-year effectiveness of IDegLira were assessed. |
What was learned from the study? |
IDegLira represents an effective, safe, and easy to manage diabetes treatment. |
IDegLira represents a good option in patients requiring intensification as well as simplification of their diabetes treatment scheme. |
Introduction
Type 2 diabetes (T2D) is a progressive disease requiring progressive treatment changes to achieve adequate glucose target [1]. On the basis of guidelines, metformin is commonly the first-line therapy, but, when metformin is no longer sufficient to maintain satisfactory glucose levels, other treatment classes including oral or injectable agents can be added [2, 3].
In the most recent guidelines [2, 3], the class of glucose-like peptide 1 receptor agonists (GLP-1 RA) plays a key role. They are recommended for treating patients with established cardiovascular disease as a first-line therapy and all other patients as a second-line therapy, as well as the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors, after metformin. On the other hand, basal insulin in association with oral agents and/or short-acting insulin can be taken into consideration at each stage of diabetes progression.
However, despite the different innovations and treatment options now available for diabetes care, many people do not reach the recommended targets [4]. Barriers to achievement of these targets are multifactorial and are summarized under the term “inertia”. From the patient perspective inertia includes fear of injections, lack of adherence to complex therapeutic schemes or polytherapy, and fear of side effects (especially hypoglycemia and weight gain) [5, 6]. The perception of these barriers is often shared also by the treating physician.
There is a strong rationale for use of combined GLP-1 RA and basal insulin owing to their synergistic effects on complementary physiopathological mechanisms underlying diabetes, targeting fasting blood glucose and postprandial glucose simultaneously [7]. Also, established and emerging indications of GLP-1 RA are also related to weight loss, cardiovascular protection, and renal benefits [8]. In addition, if GLP1-RA and basal insulin are prescribed as a fixed ratio combination (FRC) formulation, the additional advantage of a single daily injection is also added, helping patients to improve their compliance and therapy acceptance, and therefore to improve their metabolic profile [9].
It cannot be forgotten that minimizing hypoglycemia and cardiovascular risk are now considered by the modern diabetologist as key objectives of diabetes therapy, in addition to glucose control. Following the existing evidence, each diabetologist is called to proactively review current treatments and assess the need for therapy changes [2, 3].
In patients with poorly controlled diabetes, changes can have two different directions and aims: patients needing an effective and safe insulinization and patients treated with basal-bolus schemes needing to simplify their therapy to increase compliance and reduce fear of side effects [10, 11].
Among basal insulins, second-generation ones are preferred compared to first-generation analogues owing to their improved pharmacodynamic and pharmacokinetic profile [12].
On the basis of this body of evidence, our center has worked in recent years to review ongoing therapies of our patients to improve appropriateness and outcomes of care. As a result of the important information acquired by real-world evidence on the impact of new treatments on routine clinical practice [13], we designed a protocol aiming to retrospectively assess the clinical profile of patients starting the FRC of the second-generation basal insulin degludec and the GLP1-RA liraglutide (IDegLira) and its effectiveness.
IDegLira has been available on the market in the European Community since September 2014, and in Italy since October 2017. IDegLira can be prescribed in T2D as an adjunct to diet and exercise in addition to other oral medicinal products for the treatment of diabetes. IDegLira is indicated in the transfer from any prior insulin regimen that includes a basal insulin component (including multi-injection regimens). It requires a single daily administration and ensures stable glycemic profiles during the day. The IDegLira label states that it should be initiated at 10 units (10 units degludec plus 0.36 mg liraglutide) in patients transferring from oral antidiabetic drugs and at 16 units (16 units degludec plus 0.58 mg liraglutide) in patients transferring from basal insulin or a GLP-1 RA. On the other hand, the maximum dose is 50 U/day, which is equivalent to 50 U of degludec and 1.8 mg of liraglutide (the latter is the maximum approved dose for T2D treatment). IDegLira can be administered at any time during the day, regardless of meals [14].
In previous randomized and observational studies [15,16,17] involving insulin-naïve and previously insulin-treated patients, the use of IDegLira was associated with important results in terms of improved glycemic control, both in newly diagnosed and long-lasting T2D cases out of glycemic target.
The aim of the present analysis was to examine characteristics of patients who were prescribed IDegLira under routine clinical conditions, to assess therapy changes associated with the initiation of IDegLira (with a special focus on sulfonylureas that according to Italian guidelines should be interrupted and replaced with newer and safer options), titration of IDegLira, and effectiveness of IDegLira on metabolic control and additional cardiorenal parameters.
Methods
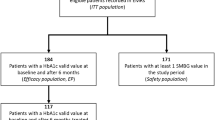
This analysis was based on a retrospective patient chart review, performed in the U.O.S.V.D. Endocrinology/Diabetology—Cittadella della Salute ASL Lecce, Italy.
Inclusion criteria were diagnosis of T2D irrespective of disease duration, male or female gender, age ≥ 18 years, treated with any glucose-lowering drug.
Exclusion criteria were other diabetes types, reduced (< 3 months) life expectancy, chronic pancreatitis, previous malignancy or diagnosis of malignancy within 3 months from the start of IDegLira treatment, acute diseases occurring within 3 months from the start of IDegLira, estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73 m2, severe liver disease, congestive heart failure New York Heart Association (NYHA) class IV.
At baseline (T0), defined as the date of the visit with the first prescription of IDegLira, the following sociodemographic and clinical information was collected: gender, age diabetes duration, previous and concomitant diabetes treatments, glycated hemoglobin (HbA1c), fasting blood glucose (FBG), weight, body mass index, eGFR, and lipid profile.
After 12 months (T12) the following clinical information was collected: HbA1c, FBG, weight, body mass index, creatinine, eGFR, lipid profile, and diabetes treatments.
The primary endpoint was the change in mean HbA1c levels after 12 months. Secondary endpoints were the change in the frequency of use of concomitant drugs from the last prescription before IDegLira initiation (T−1) to T0, and the changes of mean levels of FBG, weight, BMI, lipid profile, eGFR, and dose of IDegLira between T0 and T12.
The study protocol was approved by the “ASL Lecce” Ethics Committee, Italy (Prot. No. 4; December 14, 2022).
Statistical Analysis
Considering the descriptive nature of this analysis, a formal sample size calculation was not performed. However, a minimum sample size of 47 patients allowed one to detect, with a statistical power of 90% and a significance level (alpha) of 0.05, a decrease in HbA1c levels of at least 0.5%, assuming an estimated standard deviation of differences of 1.0.
Statistical analyses were performed overall and by stratifying analysis population by previous treatment schemes: oral hypoglycemic agents (OHAs), GLP-1 RA with or without insulin and other OHAs (GLP1-RA), basal-oral therapy (BOT), and basal-bolus (BB).
Descriptive data are summarized as mean and standard deviation for continuous variables and frequency and proportion for categorical variables. Previous and concomitant treatments according to the cohort were compared using the paired t test in case of continuous variables and the McNemar test for categorical variables. Statistical significance was declared if the p value was less than 0.05.
Changes in continuous endpoints (HbA1c, FBG, body weight, BMI, eGFR, and lipid profile) were assessed by applying mixed models for repeated measurements. This method was adopted to handle missing data by means of maximum likelihood estimation, thus allowing the estimates at each follow-up visit to be based on all initial cases. Results are expressed as estimated mean or estimated mean difference from T0 with their 95% confidence interval (95% CI). Paired t test derived from linear mixed models for repeated measurements was applied for within-group comparisons. Comparative effectiveness analysis between treatment groups was avoided because of the systematic differences in the cohorts and the small sample size.
SAS software (release 9.4) was used to perform all the analyses.
Results
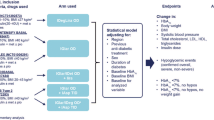
Overall, 87 patients were treated with IDegLira in the period between January 2019 and December 2021. Baseline characteristics are shown in Table 1. Patients had a mean age of 73.9 ± 9.2 years and a mean diabetes duration of 18.2 ± 6.7 years; 62.1% were male (Table 1). At the first prescription of IDegLira, elevated mean levels of HbA1c (8.3 ± 1.3%) and BMI (30.4 ± 5.5 kg/m2) were documented (Table 1). Patients initiating IDegLira were previously treated with a large array of different treatments: 21.8% with OHA only, 47.1% with BOT, 5.8% with schemes including GLP-1 RA with or without basal insulin, and 25.3% with BB (Fig. 1).
At the first prescription of IDegLira, concomitant therapy with OHA was revised: secretagogues and schemes including two or more OHA were substantially reduced, leaving metformin as the most prevalent OHA (81.6%) used in combination with IDegLira (Fig. 2). Changes in the use of classes of diabetes treatment at the first prescription of IDegLira by previous treatment scheme are reported in Supplementary Table 1.
In the OHA group, the mean starting dose of IDegLira was 18.7 ± 3.1 U; in the BOT group, the mean daily insulin dose increased from 15.5 ± 5.8 U to 20.3 ± 4.5 U; in BB group, the mean daily insulin dose decreased from 57.0 ± 22.8 U to 24.1 ± 4.4 U (Table 2).
During 12 months the mean dose of IDegLira was slightly titrated. The estimated mean dose (95% CI) at T0 was 21.09 (20.07; 22.11) U and that at T12 was 22.38 (21.02; 23.73) U, with an estimated mean change of + 1.29 (0.49; 2.08) U.
Longitudinal models showed that after 1 year, HbA1c was significantly reduced by 1.25% (95% CI − 1.48; − 1.03) (primary endpoint). As for the secondary effectiveness endpoints, during 12 months FBG was significantly reduced by 52.9 mg/dl, and body weight by 2 kg (Fig. 3). Furthermore, eGFR levels and lipid profile significantly improved (Table 3).
No patient reported severe hypoglycemia episodes after IDegLira initiation.
Discussion
In this population of insulin-naïve and previously insulin-treated patients with T2D reflecting usual care, we documented that it is feasible and clinically effective to review previous therapies and adopt a fixed ratio combination of second-generation insulin and GLP-1 RA to improve metabolic control and cardiorenal risk factors. The success of therapy was documented in spite of the mean dose of IDegLira being lower (on average 22 U) than the maximum allowed (50 U). This aspect underlines the importance of comparing the theoretical efficacy results of the trials with the effectiveness results which take into consideration not only the mechanism of action but also daily barriers such as compliance. It should also be noted that larger starting doses than those reported by the drug label were prescribed according physician judgment, on the basis of the personalized insulin needs; these starting doses required only marginal titrations during 12 months.
In Italy, 6.3% of the population is affected by diabetes. All citizens are covered by government health insurance. Primary care for diabetes is provided by general practitioners and diabetes outpatient clinics. Many classes of drugs are in the therapeutic algorithm [3] and are easily available for patients. However, many of these patients are not at target in terms of metabolic control and cardiovascular risk factors [4]. In patients on basal-bolus schemes, prevalence of patients not at target is particularly high because of the fear of hypoglycemia, poor compliance, and unavailability of a caregiver. On the other hand, there is a recognized inertia in initiating insulin in poorly controlled patients with T2D as a result of both patient and physician barriers, such as fear of hypoglycemia and weight gain and inadequate resources for treatment education [18, 19]. Flexibility and easiness of IDegLira are relevant elements for improving adherence to prescribed treatments; in this respect, IDegLira represents a good option, requiring only one daily administration. Furthermore, in patients previously treated with basal-bolus regimens, switching to IDegLira translated into a halving of the insulin dose, likely leading to a reduction of hypoglycemia risk. Hypoglycemia is not only an unpleasant experience for the patient but also an increased risk of dementia and cardiovascular complications especially in an already vulnerable population [20]. Existing data also show that hypoglycemia is a major cost for the healthcare system, quantified in Italy as over 100 million euros per year for direct and indirect costs [21].
Furthermore, while obesity is a major cardiovascular risk factor, it is known that insulin is associated with weight gain, while GLP-1 RA represents a breakthrough in the therapy of obesity [22]. We found that in patients starting IDegLira after 1 year body weight decreased by 2 kg (corresponding to a BMI reduction of 0.9 kg/m2). Additional benefits in cholesterol levels and eGFR were found, which are in line with existing knowledge about the cardiorenal benefits of GLP-1 RA [8]. In addition, at the start of IDegLira, the use of secretagogues was reduced from 49.4% to 6.9%. Use of sulfonylureas is no longer recommended in Italian guidelines [3] and prevalence of use has substantially decreased in recent years; however, at the national level 8.8% are still treated with these drugs [4]. Our approach represents an example of a strategy to facilitate the discontinuation of this class of drugs.
A previous Italian study also documented that IDegLira treatment is a valid option for patients who are failing to achieve glycemic control targets and/or struggling with the side effects of other insulin therapies, such as weight gain and hypoglycemia [23].
The study has strengths and limitations. The major strength is the use of real-world data to assess performance and adherence to the most recent treatment approaches. Major limitations are the small sample size which limits the subgroup analysis, the lack of patient-reported outcomes (not available because of the retrospective design), and the lack of information about mild or symptomatic hypoglycemia and adverse events. However, no patients interrupted the treatment as a result of lack of tolerability.
Our results also have implications. IDegLira represents an effective, safe, and easy to manage diabetes treatment both in patients intensifying and simplifying their diabetes treatment.
Conclusion
This analysis is a proof of concept that it is possible to proactively review and change suboptimal or inappropriate treatment schemes to improve clinical outcomes of poorly controlled patients with T2D. The partial interruption of other anti-hyperglycemic drugs and the initiation of once-daily administration of IDegLira suggest an association with reduction in the number of drugs to be administered every day and relevant improvements in clinical outcomes.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
18 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s13300-024-01576-9
References
Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–6. https://doi.org/10.2337/dc09-S301.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86. https://doi.org/10.2337/dci22-0034.
Mannucci E, Candido R, Monache LD, et al. Italian guidelines for the treatment of type 2 diabetes. Acta Diabetol. 2022;59:579–622. https://doi.org/10.1007/s00592-022-01857-4.
Russo G, Di Bartolo P, Candido R, et al. The AMD ANNALS: A continuous initiative for the improvement of type 2 diabetes care. Diabetes Res Clin Pract. 2023;199: 110672. https://doi.org/10.1016/j.diabres.2023.110672.
Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427–37. https://doi.org/10.1111/dom.13088.
Cucinotta D, Nicolucci A, Giandalia A, et al. Temporal trends in intensification of glucose-lowering therapy for type 2 diabetes in Italy: data from the AMD Annals initiative and their impact on clinical inertia. Diabetes Res Clin Pract. 2021;181: 109096. https://doi.org/10.1016/j.diabres.2021.109096.
Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. 2023;20:463–74. https://doi.org/10.1038/s41569-023-00849-3.
Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398:262–76. https://doi.org/10.1016/S0140-6736(21)00536-5.
Perreault L, Rodbard H, Valentine V, Johnson E. Optimizing fixed-ratio combination therapy in type 2 diabetes. Adv Ther. 2019;36:265–77. https://doi.org/10.1007/s12325-018-0868-9.
Fadini GP, Buzzetti R, Nicolucci A, et al. Comparative effectiveness and safety of glargine 300 U/mL versus degludec 100 U/mL in insulin-naïve patients with type 2 diabetes. A multicenter retrospective real-world study (RESTORE-2 NAIVE STUDY). Acta Diabetol. 2022;59:1317–1330. https://doi.org/10.1007/s00592-022-01925-9.
Rizza S, Piciucchi G, Mavilio M, et al. Effect of deprescribing in elderly patients with type 2 diabetes: iDegLira might improve quality of life. Biomed Pharmacother. 2021;144:112341. https://doi.org/10.1016/j.biopha.2021.112341.
Cheng A, Bailey TS, Mauricio D, Roussel R. Insulin glargine 300 U/mL and insulin degludec: a review of the current evidence comparing these two second-generation basal insulin analogues. Diabetes Metab Res Rev. 2020. https://doi.org/10.1002/dmrr.3329.
Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf. 2017;26:1033–9. https://doi.org/10.1002/pds.4297.
Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/xultophy-epar-product-information_en.pdf. Accessed 5 Oct 2023.
Tibaldi J, Mercado ME, Strong J. How effective is the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) in different patient populations, and when should it be used in clinical practice? Clin Diabetes. 2020;38:339–47. https://doi.org/10.2337/cd20-0014.
Price H, Blüher M, Prager R, et al. Use and effectiveness of a fixed-ratio combination of insulin degludec/liraglutide (IDegLira) in a real-world population with type 2 diabetes: results from a European, multicentre, retrospective chart review study. Diabetes Obes Metab. 2018;20:954–962. https://doi.org/10.1111/dom.13182.
Fadini GP, Buzzetti R, Fittipaldi MR, et al. IDegLira for the real-world treatment of type 2 diabetes in Italy: protocol and interim results from the REX observational study. Diabetes Ther. 2022;13:1483–97. https://doi.org/10.1007/s13300-022-01287-z.
Nicolucci A, Rossi MC. Incretin-based therapies: a new potential treatment approach to overcome clinical inertia in type 2 diabetes. Acta Biomed. 2008;79:184–91.
Nicolucci A, Kovacs Burns K, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med. 2013;30:767–77. https://doi.org/10.1111/dme.12245.
Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther. 2012;14(Suppl 1):S51–8. https://doi.org/10.1089/dia.2012.0031.
Giorda CB, Rossi MC, Ozzello O, et al. Healthcare resource use, direct and indirect costs of hypoglycemia in type 1 and type 2 diabetes, and nationwide projections. Results of the HYPOS-1 study. Nutr Metab Cardiovasc Dis. 2017;27:209–16. https://doi.org/10.1016/j.numecd.2016.10.005.
Skow MA, Bergmann NC, Knop FK. Diabetes and obesity treatment based on dual incretin receptor activation: “twincretins.” Diabetes Obes Metab. 2016;18:847–54. https://doi.org/10.1111/dom.12685.
Zenari L, Da Porto A, De Moliner L, et al. Real-world evaluation of glycemic outcomes and extra-glycemic parameters in diabetic patients treated with the combined formulation degludec-liraglutide (Ideglira). Diabetes Ther. 2021;12(1):197–209. https://doi.org/10.1007/s13300-020-00945-4.
Acknowledgements
We thank the participants of the study.
Medical Writing/Editorial Assistance
Editorial assistance (statistical analysis and medical writing) was provided by CORESEARCH SRL (Maria Chiara Rossi, Giusi Graziano) through a Novo Nordisk S.p.A. unconditional grant. The authors of the publication are fully responsible for the contents and conclusions. Novo Nordisk S.p.A. did not influence and has not been involved in the data interpretation presented in the manuscript.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article. Rapid Service Fee was also funded by Novo Nordisk S.p.A.
Author information
Authors and Affiliations
Contributions
Isabella Romano and Rosalia Serra were responsible for study concept and design of data, acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Dr. Rosalia Serra is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of Interest
Rosalia Serra has received speaker’s fee from Eli-Lilly. Isabella Romano has nothing to disclose.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The study protocol was approved by the “ASL Lecce” Ethics Committee, Italy (Prot. No. 4; December 14, 2022). Informed consent was not required by the ethics committee.
Additional information
The original online version of this article was revised due to update in Introduction text.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Romano, I., Serra, R. Use of IDegLira to Intensify, Simplify, and Increase Appropriateness of Type 2 Diabetes Therapy: A Real-Life Experience. Diabetes Ther 15, 145–154 (2024). https://doi.org/10.1007/s13300-023-01489-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01489-z