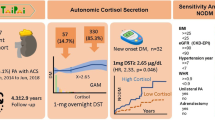
Abstract
Introduction
An increased midnight cortisol (MC) has been described in end-stage kidney disease (ESKD) and type 1 diabetes (T1D). Lower circulating levels of the cytokine soluble tumor necrosis factor (TNF)-like weak inducer of apoptosis (sTWEAK) have been found in T1D and ESKD and associated with cardiovascular (CV) events in the latter. We aimed to study MC and sTWEAK in simultaneous pancreas-kidney transplant (SPKT) recipients, and the association of these markers with CV risk factors and transplant outcomes.
Methods
This was a retrospective cohort study including subjects with T1D who received a first SPKT between 2008 and 2020. MC and sTWEAK at baseline were correlated with CV risk factors and evolution 1 year after SPKT.
Results
We included 29 subjects (58.6% women, mean age 43.5 ± 7.5 years, diabetes duration 31.9 ± 9.4 years). Systolic blood pressure (SBP) increased directly with MC quartiles, despite similar hypertension prevalence (p < 0.05). At 1 year, antihypertensive treatment was deintensified in those in lower MC quartiles (p < 0.05). Diabetic neuropathy prevalence decreased progressively in higher cortisol quartiles (p for trend = 0.005). Low MC was associated with delayed kidney graft function (p for trend = 0.044), and high sTWEAK with kidney graft rejection (p for trend = 0.018). In multivariate analyses, MC (standardized-β 0.505, p = 0.004) and age (standardized-β − 0.460, p = 0.040) were independently correlated with SBP, and MC was independently associated with the presence of diabetic neuropathy (OR 0.633, 95% CI 0.425–0.944, p = 0.025), adjusted for confounders.
Conclusions
In this exploratory study, lower MC was associated with a lower baseline SBP, an improvement of antihypertensive treatment 1 year after transplant, and a higher diabetic neuropathy prevalence in SPKT recipients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Increased midnight cortisol has been described both in advanced stages of chronic kidney disease and in type 1 diabetes and associated with cardiovascular risk factors such as increased systolic blood pressure in the latter. |
Circulating soluble tumor necrosis factor (TNF)-like weak inducer of apoptosis (sTWEAK) regulates inflammation and insulin resistance in adipose tissue; low levels have been described in chronic kidney disease and in type 1 diabetes and have been associated with cardiovascular events in the former. |
Increased midnight cortisol and decreased sTWEAK levels may be associated with a worse cardiovascular profile in people with type 1 diabetes and end-stage chronic kidney disease undergoing simultaneous pancreas-kidney transplant. |
What was learned from the study? |
A lower midnight cortisol was associated with a lower baseline systolic blood pressure, an improvement of antihypertensive treatment 1 year after transplant, and a higher diabetic neuropathy prevalence in this population; additionally, midnight cortisol and sTWEAK have been associated with transplant complications. |
The role of the hypothalamus pituitary adrenal axis and inflammatory cytokines in the development and changes in blood pressure and diabetic neuropathy in simultaneous pancreas-kidney transplant recipients warrants further study. |
Introduction
Type 1 diabetes (T1D) is a condition with a great cardiovascular burden; cardiovascular disease (CVD) is still the leading cause of morbidity and mortality in these subjects [1]. Likewise, chronic kidney disease (CKD), often comorbid with diabetes, hypertension, and other metabolic complications, is an independent risk factor for CVD and premature mortality [2]. Hence, the coexistence of both conditions dramatically increases cardiovascular (CV) risk and mortality [3]. Simultaneous pancreas-kidney transplantation (SPKT) is a currently established treatment for patients with diabetes and end-stage CKD (ESKD). SPKT stabilizes or improves diabetes chronic complications and dialysis-related morbidity, improving life expectancy [4]. Still, CVD risk remains high, especially in those with early pancreas and/or kidney graft failure [5].
Hypercortisolism is associated with an increased CV risk [6]. In CKD, functional disturbances in the hypothalamus pituitary adrenal axis (HPA) have been described, which become more pronounced as kidney impairment progresses [7]. Patients with ESKD on dialysis present an increased midnight cortisol (plasmatic and salivary) and a resistance to cortisol suppression after dexamethasone [8, 9]. The mechanisms and possible consequences of these disturbances remain unknown. Some studies have suggested that the activation of the HPA axis in subjects with type 2 diabetes could lead to CKD development and progression through an increased intracellular cortisol action [10,11,12]. In T1D, age, central obesity, increased systolic blood pressure (SBP), sedentarism, and smoking habit have been associated with increased midnight salivary cortisol [13,14,15]. Besides, an impaired stress-related adaptation of the HPA axis [16] and an impaired cortisol metabolism [17] have also been described in young subjects with T1D. In kidney transplant (KT) recipients, differential expression of glucocorticoid regulating and receptor genes could discriminate between patients with functional tolerance from those with chronic rejection [18]. Finally, suppressed HPA-axis activity is associated with higher prevalence of metabolic syndrome in prednisolone-treated KT recipients [19].
Circulating soluble tumor necrosis factor (TNF)-like weak inducer of apoptosis (sTWEAK) is a cytokine that regulates inflammation and insulin resistance in adipose tissue. Lower sTWEAK concentrations, indicating ongoing inflammation, have been found in hemodialysis and KT recipients [20] and have been associated with atherosclerotic burden and atheromatosis progression as well as cardiovascular events (CVE) in CKD [21, 22]. sTWEAK has also been described to be decreased in T1D [23]. No studies have evaluated the HPA axis or sTWEAK in subjects with T1D and CKD or SPKT recipients.
Therefore, we aimed to study midnight serum cortisol (MC) in subjects with T1D and ESKD undergoing SPKT, its association with cardiovascular risk factors at baseline and after SPKT, as well as its relation to SPKT complications. As a secondary outcome, we also aimed to study sTWEAK and its association with MC and cardiovascular risk factors.
Methods
Study Design and Participant Selection
We conducted a single-center retrospective cohort study. Patients with T1D who received a first SPKT between 2008 and 2020 were included. Data were collected at transplant and during the first year after SPKT. The study protocol was conducted according to the principles of the Declaration of Helsinki and approved by the institution’s research ethics committee.
All participants were diagnosed with T1D, ascertained by experienced endocrinologists, on the basis of specific pancreas antibodies (glutamic acid decarboxylase and/or tyrosine-phosphatase-like protein IA2 antibodies), abrupt onset of the hyperglycemia, and/or the need for continuous insulin treatment from the beginning. Those with ESKD (CKD stages IV–V [estimated glomerular filtration rate [eGFR] < 20 mL/min/1.73 m2)] received SPKT. Exclusion criteria were type 2 diabetes (T2D) or increased pretransplant C-peptide levels (> 3 ng/mL), previous or active glucocorticoid or immunosuppressive treatment, morbid obesity (body mass index [BMI] ≥ 40 kg/m2), drugs interfering with the HPA axis, or previous solid organ transplant. In particular, all subjects with active or past steroid use were excluded from the study.
All patients followed a standardized multidisciplinary pretransplant evaluation and follow-up, which included the assessment of graft function and the evaluation of pre-existent diabetes complications [24].
To ascertain the association of midnight cortisol on the cardiovascular risk profile and SPKT evolution, only those subjects who had a pretransplant blood extraction between 11:00 pm and 1:00 am were selected. A total of 29 subjects were identified and included.
Clinical and Laboratory Measures
Demographic and clinical variables such as age, sex, smoking habit, cardiovascular comorbidities, cardioprotective drugs, previous CV disease, and history of diabetes complications were obtained from medical records.
Physical examination included weight and height (calculating the BMI, accordingly), blood pressure measured after a 5-min supine rest, and following 3 min of quiet standing, ankle brachial index (ABI; using a standardized protocol [25]), vibration perception threshold measured in both inferior limbs by a biothesiometer (Bio Medical Instrument Co, Newbury, OH), and exhaustive examination of the feet. All the procedures were performed by trained nurses.
The presence of diabetic retinopathy was always ascertained and graded by an ophthalmologist. Diabetic neuropathy was evaluated by symptoms, an abnormal vibration perception threshold value (≥ 25 V; measured by a biothesiometer bilaterally on the protuberance of the first toes and on the spine of the tibias), or the presence of orthostatic hypotension [a reduction of ≥ 20 mmHg in SBP or ≥ 10 mmHg in diastolic blood pressure (DBP) after 5 min of supine rest and following 3 min of quiet standing]. Peripheral arterial disease was evaluated by symptoms and history of revascularization or amputation, or ABI < 0.9 [26]. Ischemic heart disease was evaluated by previous history of myocardial infarction, angina, history of revascularization or positive stress test, and cerebrovascular disease by previous history of ischemic or hemorrhagic stroke or transient ischemic attack. The complete pretransplant work-up has been thoroughly described previously [5, 27].
Standardized assays were used to measure glucose, HbA1c, lipid profile (including total cholesterol, HDL-cholesterol, triglycerides; LDL-cholesterol [LDL-c] was calculated with the Friedewald formula), C-peptide, and creatinine and urinary albumin-to-creatinine ratio in the local laboratory. The eGFR was obtained with the CKD Epidemiology Collaboration equation (CKD-EPI).
A blood test was performed between 11:00 pm and 1:00 am on the night before SPKT to measure midnight cortisol, cortisol-binding globulin (CBG), and sTWEAK. All blood samples from 2008 till 2020 were measured in the hormonal laboratory in the institution as follows: serum cortisol was measured by chemiluminescence immunoassay (Atellica IM1600, Siemens Healthineers, Tarrytown, NY, USA), CBG was measured by radioimmunoassay (DiaSource, Louvain-la-Neuve, Belgium), and sTWEAK was measured using enzyme-linked immunosorbent assay (kit BMS2006INST, Bender MedSystems, Burlingame, California).
Transplant Outcomes
Pancreas graft failure was defined as any of the following: (a) graft removal, (b) C-peptide < 1 ng/mL, (c) total daily insulin dose > 0.5 U/kg, or (d) patient death. Pancreas early graft failure (EGF) was defined as any pancreas graft failure during the first 90 days following SPKT. Kidney graft failure was defined as return to dialysis, retransplantation, or patient death. Kidney delayed graft function was defined as the need for at least one session of hemodialysis during the first week following SPKT.
Cardiovascular events (CVE) in the year following SPKT were registered, including cardiac, cerebrovascular, or peripheral arterial disease, as described above.
Statistical Analyses
Data are presented as median and 25th and 75th percentiles, mean ± SD for non-normal and normal distributions, respectively, or number (percentage). Normal distribution of continuous variables was evaluated with the Kolmogorov–Smirnov test.
The cohort was divided according to MC and sTWEAK quartiles. Then, multiple-group analyses in clinical and laboratory variables were performed using analysis of variance (ANOVA), Kruskal–Wallis, and chi-square tests as appropriate. Bonferroni and Jonckheere–Terpstra tests were performed to assess linear trends, for parametric and non-parametric variables, respectively. The differences between baseline and 1-year follow-up in cardiovascular risk factors were assessed with paired tests (Student’s t test and Wilcoxon test for continuous variables; McNemar’s test for categorical variables) for all the cohort and according to MC quartiles.
To explore for independent relationships between MC (independent variable) and baseline SBP (dependent variable) a logistic binary regression multivariable model was constructed. The model included age, sex, BMI, eGFR, and diabetes duration. A logistic regression analysis model was also constructed to explore for independent relationships between MC (independent variable) and diabetic neuropathy (dependent variable), including age, sex, SBP, sTWEAK, and diabetes duration as co-variables.
Significance level was defined as a p value < 0.05. IBM SPSS Statistics 23.0 (SPSS, Inc; Chicago, Illinois) was used to perform the statistical analysis.
Similar methods have been presented elsewhere [28].
Results
Study Population Characteristics
A total of 29 subjects were included: 58.6% women, mean age at transplant 43.5 ± 7.5 years. Diabetes duration was 31.9 ± 9.4 years, 82.8% were on hemodialysis or peritoneal dialysis, for 2.5 ± 1 years before SPKT. The prevalence of hypertension and dyslipidemia was 93.1% and 71.4%, respectively. All patients had diabetic retinopathy, 62.1% had neuropathy, and 24.1% a previous cardiovascular event. Mean MC was 8.9 ± 5.2 μg/dL. The overall characteristics of the study population are shown in Table 1.
Cardiovascular Risk and Diabetic Complications According to Midnight Cortisol Quartiles
Subjects in the third quartile were older than the remaining population (p = 0.006). SBP was progressively higher with increasing cortisol quartiles, despite similar hypertension prevalence; LDL-c also progressively increased proportionally to cortisol quartiles (p for all < 0.05). On the other hand, prevalence of diabetic neuropathy decreased progressively with increasing cortisol quartiles (p for trend = 0.005). A marginally significative trend for an increased prevalence of smokers in the superior quartile was observed (p = 0.070). No differences according to sex, BMI, CVD events, or other comorbidities or biochemical parameters were seen. CBG and sTWEAK levels were comparable across groups (Table 1).
Logistic regression models were created to ascertain the independent association of MC with cardiovascular risk factors and pretransplant diabetic neuropathy. MC (β 2.483, standardized-β 0.505, p = 0.004) and age (β − 1.237, standardized-β − 0.460, p = 0.040) were independently correlated with SBP, adjusted for BMI, eGFR, diabetes duration, and time on dialysis.
Additionally, MC was independently associated with the presence of pretransplant diabetic neuropathy (OR 0.633, 95% CI 0.425–0.944, p = 0.025) adjusted for age, sex, systolic blood pressure, sTWEAK, and diabetes duration (Fig. 1).
Midnight Cortisol and Transplant-Related Outcomes
Patients with MC in the lower half had higher incidence of delayed kidney graft function, compared to none in the higher quartiles (p for trend = 0.044). A trend to a higher prevalence of early pancreas dysfunction in lower quartiles was also seen (p for trend = 0.094).
No differences on immunosuppressive treatment, graft rejection, surgical complications rates, or CVE at 1 year after SPKT were seen (Table 2).
Midnight Cortisol Quartiles and Cardiovascular Risk at 1 Year
One year after SPKT, normoglycemia was restored in all but the third quartile, whose average was in the prediabetic range, despite no differences in early graft failure incidence. However, this group had a higher pre-SPKT HbA1c. Insulin secretion was also restored in all study groups, albeit C-peptide at 1 year was lower in the fourth quartile. Kidney function was restored in all study groups. Triglycerides improved significantly in the superior quartile, the group with higher pre-SPKT triglycerides levels. No significant differences in weight were observed.
Regarding cardioprotective treatment, a decrease in antihypertensive and lipid-lowering treatment was observed in all the cohort, despite similar LDL-c levels. In the subgroup analysis, the antihypertensive treatment deintensification was observed only in the two lower quartiles; the lipid-lowering deintensification did not differ across study groups (Table 3).
sTWEAK and Transplant-Related Outcomes
All subjects with kidney graft rejection were in the two upper sTWEAK quartiles, mostly in the fourth quartile (p for trend = 0.018). No differences in immunosuppressive therapy were found (Table 4).
sTWEAK and 1-Year Cardiovascular Risk and Diabetic Complications
The proportion of women was greater in the upper sTWEAK quartiles (p for trend = 0.037). This association was lost after adjusting for confounders (age, BMI, diabetes duration, time in dialysis).
Weight at SPKT and at 6 and 12 months was proportionally lower in increasing sTWEAK quartiles (p for trend = 0.023, 0.005, and 0.023; respectively), albeit without differences in BMI. In multivariate regression analysis (adjusted for midnight cortisol, sTWEAK, age, eGFR, and time on dialysis), BMI (β 2.989, standardized-β 0.879, p < 0.001), sex (β − 10.433, standardized-β − 0.377, p = 0.001), and diabetes duration (β − 0.534, standardized-β − 0.362, p = 0.007) were independently correlated with weight at SPKT. Multivariate analyses of weight at 6 and 12 months depicted similar results.
All subjects with cerebrovascular disease at transplant were in the first sTWEAK quartile (p for trend = 0.047) and a higher proportion of patients with peripheral arterial disease were also found in the first quartile (p = 0.013). A marginally significative trend for a longer CKD duration with increasing quartiles was observed (p for trend = 0.066). There were no differences in other cardiovascular risk factors, lipid profile, or other diabetic complications (Table 4). These associations were lost when adjusted for confounders.
Discussion
To our best knowledge, this is the first study to analyze midnight serum cortisol and sTWEAK in subjects with T1D and ESKD receiving SPKT. We describe an association of MC at SPKT with cardiovascular risk factors and diabetic complications, namely an increased (resting and after standing) SBP and LDL-c and a lower diabetic neuropathy prevalence at transplant. Additionally, a lower MC was associated with an improvement in antihypertensive treatment 1 year after SPKT and higher prevalence of delayed kidney graft function. We also describe an association of sTWEAK with sex and lower weight (at baseline and 6 and 12 months after SPKT), which were lost when adjusting for confounders. All subjects with cerebrovascular disease and most subjects with peripheral artery disease had a low sTWEAK. On the contrary, subjects with kidney graft rejection had high sTWEAK levels.
T1D and CKD are both conditions which entail a great cardiovascular burden, their coexistence dramatically increasing cardiovascular risk and mortality [3]. SPKT recipients have a high prevalence of cardiovascular comorbidities and diabetic complications [27]. SPKT ameliorates diabetes chronic complications and dialysis-related morbidity and mortality [4] but cardiovascular risk remains high [5].
A few studies have described changes in cortisol regulation in CKD, especially in ESKD, as an increased midnight (salivary and serum) cortisol or a resistance to suppression with dexamethasone [8, 9]. The mechanisms and the possible implications in kidney disease progression remain largely unknown.
Some studies in T2D have suggested that the activation of the HPA axis could lead to CKD development and progression through an increased intracellular cortisol action [10,11,12]. In a study in a CKD cohort with hypertension, serum cortisol (8:00 am) was negatively associated with eGFR and positively associated with CKD markers; higher cortisol tertiles were also associated with worse renal function [29]. Another study described a functional deficiency of 11-beta-hydroxysteroid dehydrogenase type 2 (the enzyme that inactivates cortisol to cortisone in the kidney to prevent the activation of the mineralocorticoid receptor) both in children with CKD and essential hypertension, measured through the tetrahydrocortisol-to-tetrahydrocortisone urinary ratio. This was negatively correlated with eGFR and positively with SBP and DBP [30]. One study in hemodialysis patients found a correlation of high serum cortisol with a state of inflammation and higher mortality [31]. In a recent study in hemodialysis patients, high cortisol measured before a dialysis session was associated with CVD and mortality, and an oxidized LDL-c predicted an elevated serum cortisol [32]. In T1D, an increased midnight salivary cortisol has been associated with increased SBP, creatinine, and other cardiovascular risk factors and metabolic syndrome features [13].
In line with previous studies, we describe an independent association of midnight serum cortisol with high SBP. Further, improvement in blood pressure control at 1 year, indirectly assessed through antihypertensive treatment deintensification, was only observed in subjects with low midnight cortisol. In a previous study in renal transplant patients on chronic prednisolone treatment, decreased 24-h urinary cortisol excretion, as a measurement of prednisolone-related HPA axis suppression, was independently associated with metabolic syndrome and its components, such as antihypertensive treatment [19]. This has not been previously studied in SPKT recipients.
We also describe an association of midnight cortisol with higher LDL-c, but this association was lost after adjusting for confounders. In T1D, midnight salivary cortisol has been related to metabolic syndrome features but no association with LDL-c has been found [13]. Regarding CKD, oxidized LDL-c predicted an elevated serum cortisol in one study in hemodialysis patients [32].
Moreover, we observed an inverse independent association of MC with diabetic neuropathy prevalence. This could be explained by a decreased cortisol response in subjects with diabetic neuropathy. Previous literature regarding cortisol changes in diabetic neuropathy is contradictory. A classic study described impaired sympathetic activity, growth hormone, and cortisol responses to exercise (lower levels of cortisol after exercise) in subjects with diabetic autonomic neuropathy compared to subjects with diabetes without neuropathy and controls [33]. A more recent study described impaired glucagon, catecholamine, growth hormone, and cortisol responses to hypoglycemia in subjects with T1D, which were impaired to a greater extent in those T1D with autonomic neuropathy [34]. A study analyzing response to intravenously administered noradrenaline reported no differences in cortisol response between healthy volunteers and subjects with diabetes with and without autonomic neuropathy [35]. On the other hand, one study described increased cortisol and adrenocorticotropic hormone (ACTH) secretion (area under the curve) in the 8:00 am–7:00 pm period in subjects with symptomatic diabetic polyneuropathy, with a maintenance of the circadian rhythm, suggesting an increased HPA activity in these patients [36]. In a previous study, we describe an independent association of diabetic neuropathy with pancreas graft function and CVD after pancreas transplantation [37]. So, diabetic neuropathy could identify a subpopulation of subjects with worse cardiovascular profile. Whether HPA axis plays a role in this high CVD risk should be further assessed.
The cytokine sTWEAK regulates inflammation and insulin resistance in adipose tissue. It plays a beneficial role in tissue repair after acute injury, but persistent sTWEAK activation has shown an important role in pathological remodelling underlying CVD [38]. Lower sTWEAK levels, which translate ongoing inflammation, have been described in T1D [23], hemodialysis and KT recipients [20], and have been associated with subclinical atherosclerotic disease and CVE in CKD [21, 22].
In our study, the lower sTWEAK quartile grouped the two subjects with cerebrovascular disease and a higher proportion of subjects with peripheral arterial disease. This finding is consistent with previous evidence. Various articles have described an inverse correlation of sTWEAK with atherosclerosis and CVE in CKD [21, 22, 39]. In particular, one study observed lower sTWEAK levels in advanced CKD compared to earlier stages; the lowest levels were found in subjects who developed a CVE (ischemic CVD, cerebrovascular and peripheral artery disease) [40].
Regarding, transplant outcomes, we describe an inverse association of MC with delayed kidney graft function (all subjects with this complication were found in the two lower quartiles). One study described that addition of methylprednisolone to the perfusion preserving the kidney graft enhanced graft survival [41]. We hypothesize that activation of the HPA axis could have an anti-inflammatory effect, but this needs be further investigated.
We also describe an association of sTWEAK with kidney graft rejection (all subjects were in the two upper quartiles). sTWEAK has not been studied in the context of solid organ graft rejection. Nevertheless, inhibition of the TWEAK/fibroblast growth factor-inducible 14 (Fn14) system reduced transplantation-induced intestinal cell death in graft-versus-host disease after hematopoietic stem cell transplantation. The suggested mechanism was the protection of intestinal cells from TNF-induced apoptosis and not the immune response, as cytokine production or infiltration of donor T cells remained unaffected [42]. Regarding kidney damage, an increase in sTWEAK has been described 180 days following kidney transplant, paralleled by an improvement in flow-mediated dilatation, an indirect measurement of endothelial function [43]. The sTWEAK pathway has also been identified as a critical contributor to calcineurin inhibitor toxicity of the kidneys [44]. Further studies are needed to confirm this association and possible mechanisms.
Some limitations of this study have to be acknowledged. First, its observational design precludes drawing conclusions on causality. Second, the small sample size and the division of the cohort into quartiles could limit statistical power. Third, the sample for measuring MC was obtained upon admission of the patients for transplant. Stress related to the admission or the procedure could affect the HPA axis activity. Nevertheless, we believe this effect to be minimal as all subjects were in the same hospital setting. Fourth, HPA axis evaluation was incomplete as no other biochemical tests were performed: 24-h urinary cortisol was not measured as most patients had little or no residual diuresis. Besides, morning cortisol and ACTH were not measured and dynamic testing was not performed. Finally, the relatively short follow-up (1 year) limits the detection of changes in cardiovascular risk factors and transplant complications in the longer term.
Conclusion
In this exploratory study, lower midnight cortisol was associated with a lower baseline systolic blood pressure, an improvement of antihypertensive treatment 1 year after transplant, and a higher diabetic neuropathy prevalence in SPKT recipients. Additionally, midnight cortisol and sTWEAK have been associated with transplant complications. This is the first study to assess midnight cortisol and sTWEAK in this population. Further long-term studies are needed to confirm the relevance of this finding on transplant and CV outcome.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Harjutsalo V, Pongrac Barlovic D, Groop P-H. Long-term population-based trends in the incidence of cardiovascular disease in individuals with type 1 diabetes from Finland: a retrospective, nationwide, cohort study. Lancet Diabetes Endocrinol. 2021;8587:1–11. https://doi.org/10.1016/s2213-8587(21)00172-8.
GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. https://doi.org/10.1016/S0140-6736(16)31012-1.
Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:273–80. https://doi.org/10.1053/j.ackd.2014.03.003.
Fridell JA, Stratta RJ, Gruessner AC. Pancreas transplantation: current challenges, considerations, and controversies. J Clin Endocrinol Metab. 2023;108(3):614–23.
Montagud-Marrahi E, Molina-Andújar A, Pané A, et al. Impact of simultaneous pancreas-kidney transplantation on cardiovascular risk in patients with diabetes. Transplantation. 2021. https://doi.org/10.1097/TP.0000000000003710.
Vega-Beyhart A, Iruarrizaga M, Pané A, et al. Endogenous cortisol excess confers a unique lipid signature and metabolic network. J Mol Med. 2021;99:1085–99. https://doi.org/10.1007/s00109-021-02076-0.
de Luján Cardoso EM, Arregger AL, Budd D, Zucchini AE, Contreras LN. Dynamics of salivary cortisol in chronic kidney disease patients at stages 1 through 4. Clin Endocrinol (Oxf). 2016;85:313–9. https://doi.org/10.1111/cen.13023.
Wallace EZ, Rosman P, Toshav N, Sacerdote A, Balthazar A. Pituitary-adrenocortical function in chronic renal failure: studies of episodic secretion of cortisol and dexamethasone suppressibility. J Clin Endocrinol Metab. 1980;50:46–51. https://doi.org/10.1210/jcem-50-1-46.
Raff H, Trivedi H. Circadian rhythm of salivary cortisol, plasma cortisol, and plasma ACTH in end-stage renal disease. Endocr Connect. 2012;2:23–31. https://doi.org/10.1530/ec-12-0058.
Asao T, Oki K, Yoneda M, Tanaka J, Kohno N. Hypothalamic-pituitary-adrenal axis activity is associated with the prevalence of chronic kidney disease in diabetic patients. Endocr J. 2016;63:119–26. https://doi.org/10.1507/endocrj.EJ15-0360.
Gant CM, Minovic I, Binnenmars H, et al. Lower renal function is associated with derangement of 11-β hydroxysteroid dehydrogenase in type 2 diabetes. J Endocr Soc. 2018;2:609–20. https://doi.org/10.1210/JS.2018-00088.
Sagmeister MS, Taylor AE, Fenton A, et al. Glucocorticoid activation by 11β-hydroxysteroid dehydrogenase enzymes in relation to inflammation and glycaemic control in chronic kidney disease: a cross-sectional study. Clin Endocrinol (Oxf). 2019;90:241–9. https://doi.org/10.1111/cen.13889.
Melin EO, Hillman M, Landin-Olsson M. Midnight salivary cortisol secretion associated with high systolic blood pressure in type 1 diabetes. Endocr Connect. 2019;8:1520–8. https://doi.org/10.1530/EC-19-0407.
Melin EO, Hillman M, Thunander M, Landin-Olsson M. Midnight salivary cortisol secretion and the use of antidepressants were associated with abdominal obesity in women with type 1 diabetes: a cross sectional study. Diabetol Metab Syndr. 2019;11:1–12. https://doi.org/10.1186/s13098-019-0481-3.
Melin EO, Thunander M, Landin-Olsson M, Hillman M, Thulesius HO. Depression, smoking, physical inactivity and season independently associated with midnight salivary cortisol in type 1 diabetes. BMC Endocr Disord. 2014;14:75. https://doi.org/10.1186/1472-6823-14-75.
Sharma AN, Wigham J, Veldhuis JD. Corticotropic axis drive of overnight cortisol secretion is suppressed in adolescents and young adults with type 1 diabetes mellitus. Pediatr Diabetes. 2014;15:444–52. https://doi.org/10.1111/pedi.12108.
Remer T, Maser-Gluth C, Boye KR, Hartmann MF, Heinze E, Wudy SA. Exaggerated adrenarche and altered cortisol metabolism in type 1 diabetic children. Steroids. 2006;71:591–8. https://doi.org/10.1016/j.steroids.2006.02.005.
Christakoudi S, Runglall M, Mobillo P, et al. Steroid regulation: an overlooked aspect of tolerance and chronic rejection in kidney transplantation. Mol Cell Endocrinol. 2018;473:205–16. https://doi.org/10.1016/j.mce.2018.01.021.
De Vries LV, De Jong WHA, Touw DJ, et al. Twenty-four hour urinary cortisol excretion and the metabolic syndrome in prednisolone-treated renal transplant recipients. Steroids. 2017;127:31–9. https://doi.org/10.1016/j.steroids.2017.09.001.
Eskandari Naji H, Ghorbanihaghjo A, Argani H, et al. Serum sTWEAK and FGF-23 levels in hemodialysis and renal transplant patients. Int J Organ Transplant Med. 2017;8:110–6.
Fernández-Laso V, Méndez-Barbero N, Valdivielso JM, et al. Soluble TWEAK and atheromatosis progression in patients with chronic kidney disease. Atherosclerosis. 2017;260:130–7. https://doi.org/10.1016/j.atherosclerosis.2017.03.043.
Bozic M, Méndez-Barbero N, Gutiérrez-Muñoz C, et al. Combination of biomarkers of vascular calcification and sTWEAK to predict cardiovascular events in chronic kidney disease. Atherosclerosis. 2018;270:13–20. https://doi.org/10.1016/j.atherosclerosis.2018.01.011.
Llauradó G, González-Clemente J-M, Maymó-Masip E, Subías D, Vendrell J, Chacón MR. Serum levels of TWEAK and scavenger receptor CD163 in type 1 diabetes mellitus: relationship with cardiovascular risk factors. A case–control study. PLoS One. 2012;7:e43919. https://doi.org/10.1371/journal.pone.0043919.
Amor AJ, Casas A, Pané A, et al. Weight gain following pancreas transplantation in type 1 diabetes is associated with a worse glycemic profile: a retrospective cohort study. Diabetes Res Clin Pract. 2021;179:109026. https://doi.org/10.1016/j.diabres.2021.109026.
Aboyans V, Ricco J-B, Bartelink M-LEL, et al. ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2017;2018(39):763–816. https://doi.org/10.1093/eurheartj/ehx095.
Aboyans V, Ricco J-B, Bartelink M-LEL, et al. ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J. 2017;2018(39):763–816. https://doi.org/10.1093/eurheartj/ehx095.
Ruiz S, Amor AJ, Pané A, et al. Cardiovascular risk factors and cardiovascular disease in patients with type 1 diabetes and end-stage renal disease candidates for kidney-pancreas transplantation: trends from 1999 to 2017. Diabetes Res Clin Pract. 2020. https://doi.org/10.1016/j.diabres.2020.108135.
Montagud-Marrahi E, Molina-Andújar A, Pané A, et al. Impact of simultaneous pancreas-kidney transplantation on cardiovascular risk in patients with diabetes. Transplantation. 2022;106:158–66. https://doi.org/10.1097/TP.0000000000003710.
Li X, Xiang X, Hu J, et al. Association between serum cortisol and chronic kidney disease in patients with essential hypertension. Kidney Blood Press Res. 2016;41:384–91. https://doi.org/10.1159/000443435.
Mongia A, Vecker R, George M, et al. Role of 11βHSD type 2 enzyme activity in essential hypertension and children with chronic kidney disease (CKD). J Clin Endocrinol Metab. 2012;97:3622–9. https://doi.org/10.1210/jc.2012-1411.
Gracia-Iguacel C, González-Parra E, Egido J, et al. Cortisol levels are associated with mortality risk in hemodialysis patients. Clin Nephrol. 2014;82:247–56. https://doi.org/10.5414/cn108311.
Kim J, Yun K-S, Cho A, et al. High cortisol levels are associated with oxidative stress and mortality in maintenance hemodialysis patients. BMC Nephrol. 2022;23:98. https://doi.org/10.1186/s12882-022-02722-w.
Hilsted J, Galbo H, Christensen NJ. Impaired responses of catecholamines, growth hormone, and cortisol to graded exercise in diabetic autonomic neuropathy. Diabetes. 1980;29:257–62. https://doi.org/10.2337/diab.29.4.257.
Meyer C, Grossmann R, Mitrakou A, et al. Effects of autonomic neuropathy on counterregulation and awareness of hypoglycemia in type 1 diabetic patients. Diabetes Care. 1998;21:1960–6. https://doi.org/10.2337/diacare.21.11.1960.
Dejgaard A, Andersen P, Hvidberg A, Hilsted J. Cardiovascular, metabolic, and hormonal responses to noradrenaline in diabetic patients with autonomic neuropathy. Diabet Med. 1996;13:983–9. https://doi.org/10.1002/(SICI)1096-9136(199611)13:11%3c983::AID-DIA271%3e3.0.CO;2-7.
Tsigos C, Young RJ, White A. Diabetic neuropathy is associated with increased activity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 1993;76:554–8. https://doi.org/10.1210/jcem.76.3.8383141.
Boswell L, Ventura-Aguiar P, Alejaldre A, et al. Diabetic neuropathy is independently associated with worse graft outcomes and incident cardiovascular disease after pancreas transplantation: a retrospective cohort study in type 1 diabetes. Transplantation. 2023;107:475–84. https://doi.org/10.1097/TP.0000000000004275.
Blanco-Colio LM. TWEAK/Fn14 axis: a promising target for the treatment of cardiovascular diseases. Front Immunol. 2014;5:3. https://doi.org/10.3389/fimmu.2014.00003.
Valdivielso JM, Coll B, Martín-Ventura JL, et al. Soluble TWEAK is associated with atherosclerotic burden in patients with chronic kidney disease. J Nephrol. 2013;26:1105–13. https://doi.org/10.5301/jn.5000245.
Hassan SB, El-demery AB, Ahmed AI, Abukhalil RE. Soluble TWEAK and cardiovascular morbidity and mortality in chronic kidney disease patients. Arab J Nephrol Transplant. 2012;5:27–32.
McCabe RE, Lattes CG, Lorieo DR, Hashim GA, Fitzpatrick HF. The protective effect of methyl prednisolone on the machine-preserved kidney. Am Surg. 1980;46:335–9.
Chopra M, Brandl A, Siegmund D, et al. Blocking TWEAK-Fn14 interaction inhibits hematopoietic stem cell transplantation-induced intestinal cell death and reduces GVHD. Blood. 2015;126:437–44. https://doi.org/10.1182/blood-2015-01-620583.
Yilmaz MI, Sonmez A, Saglam M, et al. Soluble TWEAK plasma levels increase after renal transplantation and associate with the improvement of endothelial function. Eur J Clin Investig. 2013;43:1250–7. https://doi.org/10.1111/eci.12166.
Claus M, Herro R, Wolf D, et al. The TWEAK/Fn14 pathway is required for calcineurin inhibitor toxicity of the kidneys. Am J Transplant. 2018;18:1636–45. https://doi.org/10.1111/ajt.14632.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Laura Boswell, Pedro Ventura-Aguiar, Antonio J. Amor and Felicia A. Hanzu contributed to the study concept and design. Laura Boswell, Antonio J. Amor and Felicia A. Hanzu acquired and processed data, wrote the manuscript, designed the figures and had final responsibility for the decision to submit for publication. Pedro Ventura-Aguiar and Enrique Montagud-Marrahi acquired data and critically reviewed the manuscript. Gregori Casals, Daniela Díaz-Catalan, Elisenda Banon-Maneus, María José Ramírez-Bajo, Natalia Hierro, Fritz Diekmann, Mireia Musquera, Tonet Serés-Noriega, Enric Esmatjes and Joana Ferrer-Fàbrega wrote sections of the manuscript and reviewed and edited the manuscript. Gregori Casals and Daniela Díaz-Catalan performed laboratory measurements. Pedro Ventura-Aguiar, Enrique Montagud-Marrahi, Elisenda Banon-Maneus, María José Ramírez-Bajo and Natalia Hierro obtained blood tests and participated in laboratory measurements. All authors participated in data analysis and interpretation and reviewed the final version of the manuscript. Felicia A. Hanzu is the guarantor of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Conflict of Interest
Laura Boswell, Antonio J Amor, Enrique Montagud-Marrahi, Gergori Casals, Daniela Dias-Catalan, Elisenda Banon-Maneus, Maria Jose Ramirez-Bajo, Natalia Hierro, Fritz Diekmann, Mireia, Musquera, Tonet Seres-Noriega, Enric Esmatjes, Joana Ferrer-Fabrega, Pedro Ventura-Aguiar, and Felicia A Hanzu have no competing interests. Laura Boswell received a research grant (Resident Award “Premi Fi de Residènica Emili Letang” 2019) from Hospital Clínic de Barcelona, Research, Innovation and Education Department and a research grant (“Ajut ACD per la realització del programa de doctorat 2020”) from Associació Catalana de Diabetis (ACD).
Ethical Approval
The study protocol was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments and approved by the Hospital Clínic de Barcelona Research Ethics Committee (reference number HCB/2016/0479). All subjects provided written informed consent.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Boswell, L., Amor, A.J., Montagud-Marrahi, E. et al. Midnight Cortisol is Associated with Changes in Systolic Blood Pressure and Diabetic Neuropathy in Subjects with Type 1 Diabetes Undergoing Simultaneous Kidney-Pancreas Transplantation. Diabetes Ther 15, 165–181 (2024). https://doi.org/10.1007/s13300-023-01487-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01487-1