Abstract
Introduction
Non-alcoholic fatty liver disease (NAFLD) is part of a disease spectrum ranging from steatosis to steatohepatitis (NASH), fibrosis, and cirrhosis, and when associated with metabolic syndrome (MS), and overt diabetes is defined as metabolic NAFLD (MAFLD). Some easily available, inexpensive biomarkers have been validated based on common anthropometric and laboratory parameters, including the Fatty Liver Index (FLI), the Fibrosis (FIB)-4 Score (FIB-4), and the NAFLD Fibrosis Score (NFS). In people with overweight/obesity, MS, and diabetes, the pathogenesis of fatty liver involves parameters known to be positively affected by Neo-Policaptil Gel Retard (PGR), a phytocomplex already successfully used in adolescents and adults with MS and type 2 diabetes mellitus (T2DM). This study’s primary outcome was to assess PGR's ability to improve indirect validated signs of liver steatosis and fibrosis, i.e., FLI, FIB-4, and NFS Scores; as the secondary outcome, we aimed to confirm PGR's positive effects on anthropometric parameters and lipid levels and to assess any eventually occurring cytolysis liver marker changes in patients with MS/T2DM and MAFLD/NASH.
Methods
In this spontaneous, longitudinal, single-blind, randomized clinical study, 245 outpatients with MS/T2DM were enrolled and randomized to PGR or placebo for 24 weeks. All underwent a low-calorie diet (20–25% less than the calories required to maintain current weight) and were encouraged to intensify physical activity. Fat distribution, liver fat content/fibrosis, and biochemical parameters were evaluated at baseline and after 24 weeks.
Results
Our data show for the first time in adults with MAFLD that, when added to lifestyle changes including a hypocaloric diet and intensified physical activity, PGR improves lipid and glucose metabolism-related parameters, including insulin-resistance, and significantly reduces not only visceral fat but also liver fat content and related liver fibrosis severity. The prevalence of subjects with severe steatosis (FLI > 60) significantly decreased from 95.08 to 47.53% (p < 0.001) only in the treatment group, which also displayed a significantly decreased prevalence of medium-severe cases (F3–F4) from 83.62% to 52.35% (p < 0.001) and a markedly increased prevalence of low degree cases (F0–F1) from 9.01 to 42.15% (p < 0.001).
Conclusions
The effect of PGR is related to a reduction in the post-meal blood glucose and insulin peaks. As glucose absorption (GA) directly regulates pancreatic insulin release, the attenuated insulin response is likely due to delayed GA with decreased body weight, visceral fat, and cardiovascular risk. Also, an effect on the intestinal microbiota, already documented in the animal model, cannot be excluded, especially considering the reported PGR-related shift from the Firmicutes, notoriously responsible for increased lipid gut absorption, to the Bacteroides phylum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Non-alcoholic fatty liver disease (NAFLD) represents a disease spectrum ranging from simple steatosis to steatohepatitis (NASH), fibrosis, and cirrhosis and is frequently associated to metabolic syndrome (MS) and type 2 diabetes mellitus (T2DM). |
Neo-Policaptil Gel Retard (PGR), a natural phytocomplex, has been demonstrated to be effective in reducing factors associated with MS and T2DM, such as blood sugar, cholesterol, triglycerides, and abdominal fat distribution, all factors involved in MS and T2DM and strongly linked to fat accumulation in the liver. |
Some non-invasive and validated indicators of steatosis and liver fibrosis, such as Fatty Liver Index (FLI), Fibrosis (FIB)-4 Score (FIB-4), and NAFLD Fibrosis Score (NFS), have long been used to identify and quantify their extent. |
What was learned from the study? |
This article documents for the first time how the use of PGR in subjects with fatty liver, MS, and T2DM is able to significantly reduce the number of subjects with and the entity of liver steatosis and fibrosis and to reduce the level of transaminases. |
The use of PGR represents a new possible therapeutic option in subjects with MS and T2DM complicated by high at liver, as in metabolic NAFLD (MAFLD). |
Introduction
Fatty liver includes several conditions ranging from non-alcoholic liver disease (NAFLD), steatohepatitis (NASH), liver fibrosis of different degrees up to liver cirrhosis, frequently associated with metabolic syndrome (MS) and/or over type 2 diabetes mellitus (T2DM), and in such a case defined as metabolic NAFLD (MAFLD) [1].
To define NAFLD, at least 5% of liver cells must be filled with fat (definition achieved by histological or imaging diagnosis) and independent of other toxic, infectious, or metabolic causes or excessive alcohol consumption by the patient, according to the current literature [1,2,3,4]. MAFLD is characterized by the presence of T2DM and various comorbidities such as dyslipidemia, hypertension, overweight or obesity, and others, all potent cardiovascular risk factors, as previously extensively described [1, 2, 5, 6].
Both NAFLD and MAFLD can initiate a pathway leading to necro-inflammation and fibrosis, indicators of disease progression (NASH) [7, 8] in about 40% of cases [3, 16], and are strongly related to excess triglyceride stores in the liver. They are often present in subjects with metabolic syndrome (MS) and T2DM, once again burdening patients with high cardiovascular risk [9, 10].
The histological diagnosis of NASH is characterized not only by high liver fat content but also by hepatocyte ballooning and lobular inflammation [17], which involve the risk of progression to advanced fibrosis and HCC [12, 13]. NASH is also associated with a high risk of advanced kidney disease [21], cardiovascular disease [10], and T2DM [4, 5, 9].
MAFLD variably includes a series of conditions not always present simultaneously but all clinically relevant, such as insulin resistance, visceral obesity, dyslipidemia, hypertension, overeating, sedentary lifestyle, genetic predisposition [4, 15], and microbiota alterations [16].
The progression of fibrosis is a powerful indicator of disease severity and risk of death [14, 17,18,19], and its severity is conventionally defined by the abbreviations from F0 (absent) to F4 (cirrhosis) [20], as confirmed by epidemiological studies and meta-analyses [21,22,23,24,25].
Liver biopsy remains the gold standard for NAFLD/NASH diagnostics. However, its invasive and time-consuming nature makes such a method unsuitable for large patient cohorts and even less for screening purposes [26]. On the other hand, parameters derived from ultrasound (US) scans and fibroscans may help stage NAFLD/NASH. Nevertheless, their ability to quantify liver damage is still debated [27, 28], so some easily available, inexpensive biomarkers have been validated for this purpose based on common anthropometric and laboratory parameters, including the Fatty Liver Index (FLI), Fibrosis (FIB)-4 Score (FIB-4), and the NAFLD Fibrosis Score (NFS; 21, 29,30,31). They are routinely used for many outpatients for their high predictive value quoad vitam [26].
Despite being largely used in the past, drugs and plant extracts cannot be considered effective against NAFLD and still await confirmation in the long run because of the complex pathogenetic mechanisms involved and clinical conditions associated with a high liver fat content. Indeed, especially in people with overweight/obesity and metabolic syndrome (MS), fatty liver depends on factors already shown to be positively affected by Neo-Policaptil Gel Retard® (PGR; 34, 35). The latter is a macromolecule phytocomplex covered by a European patent (no. 1679009) and based on polysaccharides endowed with the ability to reduce carbohydrate and fat absorption rates. In particular, PGR comes from high-fiber raw materials as previously described [34] and was successfully used in adolescents and adults with MS and T2DM [34, 35] at reducing circulating levels of insulin, lipids, and post-prandial triglycerides together with insulin resistance and body fat distribution. In detail, the positive effects of PGR on glucose control, insulin resistance, and body weight in adolescents and adults were not less than those of metformin, while body fat redistribution—with special reference to visceral fat—and blood lipid profile—in terms of total and high-density lipoprotein (HDL) cholesterol and triglycerides—were not affected by metformin and improved significantly in the PGR group only [34, 35]. Such findings were of particular interest considering that both treatment groups were on a hypocaloric diet (20% fewer calories than before the trial) [34, 35].
Therefore, with such premises, the present study aimed to assess PGR's ability to improve indirect signs of liver steatosis and fibrosis (primary outcome) besides confirming the reported positive PGR effects on anthropometric parameters, lipid levels, and circulating cytolysis liver markers, including transaminases and gamma-glutamyl transferase (γ-GT), in patients with MS/T2DM and MAFLD/NASH (secondary outcome).
Methods
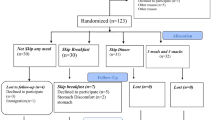
Two hundred fifty subjects with MS and T2DM were consecutively enrolled in this longitudinal, single-center, single-blind study and randomized to active treatment (treatment group; TG), taking PGR as two sachets of 2.5 g of soluble granules dissolved in water consumed before the two main mails, and control group (CG) taking placebo, similar in appearance to TG, after signing informed consent (Fig. 1). The follow-up duration was 6 months.
Ethical Approval
The Ethics Committee of the University Hospital of Campania "Luigi Vanvitelli", Naples, Italy, approved the present study (protocol no. 33478/2022, November 11, 2022). All participants signed informed consent, and data were processed anonymously according to good clinical practice guidelines. This study was conducted in conformance with good clinical practice standards. The study was carried out in accordance with the Declaration of Helsinki 1975, and subsequent amendments. All followed procedures were in accordance with the ethical standards of the responsible committee on human experimentation (both institutional and national) and in accordance with usual clinical practice. Written informed consent was obtained from all participants before enrollment.
Inclusion Criteria
-
Liver fat content > 5% (detected by ultrasonography).
-
Aspartate transferase (AST)/alanine transferase (ALT) no higher than 1.5–2 times the upper limit of normal.
-
Absence of viral, autoimmune, or toxic hepatitis.
-
Moderate or no alcohol habit.
-
Metabolic syndrome (defined according to the consensus document 2009; 9).
-
Age > 18 years for both sexes.
-
Body mass index (BMI) > 30 kg/m2.
-
Fasting glycemia (FPG) ≥ 126 mg/dl and hemoglobin A1C (HbA1c) ≥ 42 mmol/mol, or overt T2DM known for no more than 1 year (± 0.5) (American Diabetes Association [ADA] criteria 2022; 35).
-
Total cholesterol ≥ 200 mg/dl or LDL cholesterol ≥ 100 mg/dl.
-
Reliability (visiting the clinic steadily).
-
Normal estimated glomerular filtration rate (eGFR; 60–90 ml/min/1.73 m2).
-
No micro- or macro-albuminuria.
-
No previous utilization of lipid-lowering drugs against metabolic disorders.
-
Acceptance of informed consent.
Exclusion Criteria
-
Insulin treatment.
-
Previous bariatric surgery interventions.
-
Pregnancy or breastfeeding.
-
Disabling conditions, serious heart, liver, kidney, or neoplastic diseases, dementia, or inability to regularly comply with prescriptions.
-
History of drug allergy or known allergic disease.
-
Irritable bowel disease, dyspepsia, and celiac disease.
-
Any clinical condition incompatible with the study in the opinion of the investigators.
Safe limits of alcohol consumption in MAFLD were defined as alcohol consumption < 210 g per week for men and 140 g per week for women [36,37,38].
During the study, both groups of patients followed a personalized low-calorie diet ensuring 80% of current requirements (10–20% protein, 50–60% carbohydrates, and 20–30% fat) and received weekly phone calls to get motivated to continue with the diet and increase their aerobic physical activity up to at least 3 metabolic equivalents of task (METS) per week.
Similar methodologies, with the inclusion and exclusion criteria, have been described previously [32].
The general characteristics of the involved patients are shown in Table 1.
Parameters under study included: (1) body mass index (BMI), waist circumference (WC), and visceral fat percentage (% VF); (2) HbA1c and homeostatic model assessment for insulin resistance (HOMA-IR), insulin, and C-peptide; triglycerides, total cholesterol, low-density lipoprotein (LDL) cholesterol, aspartate transferase (AST), alanine transferase (ALT), gamma-glutamyl transferase (γ-GT), alkaline phosphatase (ALP), creatinine levels, and a complete blood count (automatic biochemistry analyzer with integrated system Selecta Pro XI, [Elithech, USA] and commercially available ELISA kit, as appropriate); (3) treatment adherence; (4) side effects presumably due to treatment; (5) any other adverse effects: clinical history plus circulating AST, ALT, γ-GT, alkaline phosphatase, and blood creatinine levels. Normal values were 5–55 U/l for AST, 8–48 U/l for ALT, 5–40 for γ-GT, and 40–174 IU/l for alkaline phosphatase. Venous blood samples were obtained in the morning after a 12-h overnight fast and immediately analyzed at the central laboratory of the hospital.
History of prescribed medications and their adverse reactions, anthropometry, physical examination results, and biochemical measurements were recorded for each participant at baseline and week 24. As previously described, the homeostasis HOMA-IR model was calculated to evaluate insulin resistance and islet beta-cell function, as previously described [32].
Visceral fat was measured with Body Metrix BX2000, Intela-Metrix, Inc. (Brentwood CA, US), a validated instrument based on ultrasound technology, allowing direct and immediate subcutaneous fat thickness measurement to avoid annoying and operator-dependent manual application maneuvers [39]. All parameters were measured at baseline (T-0) and after 3 (T-12) and 6 months (T-24), respectively. The indirect tests to define the presence and severity of steatosis and fibrosis for NAFLD/NASH diagnosis were:
Fatty Liver Index
FLI was calculated by the following formula, as previously defined [29]:
A FLI ≥ 60 indicates hepatic steatosis (SP = 86%; LR+ = 4.3) and < 30 rules it out (SN = 87%; LR– = 0.2), where SN = sensitivity, SP = specificity, LR+ = positive likelihood ratio, and LR– = negative likelihood ratio.
An FLI 31–59 is considered inconclusive.
FIB-4 was obtained as previously described [20, 21], with the following formula:
where a score < 2.0 = lower risk for advanced fibrosis (F3/F4) in subjects > 65 years of age and a score > 2.67 = high risk for advanced fibrosis for all ages, where F0 = no fibrosis; F1 = portal fibrosis without intralobular septa; F2 = portal fibrosis with few intralobular septa; F3 = numerous septa without cirrhosis; F4 = cirrhosis.
In NASH (non-alcoholic steatohepatitis):
-
Fibrosis F0-F1 (FIB-4 < 1.30)
-
Moderate fibrosis F2 (1.30 to 2.67)
-
Cirrhosis F3-F4 (FIB-4 >2.67)
An excellent correlation among FIB-4, Fibrotest, and elastography with histological fibrosis was demonstrated [22, 23].
NAFLD Fibrosis Score
NFS can be calculated using the following formula [21, 23]:
NAFLD fibrosis score = −1.675 + 0.037 × age (year) + 0.094 × BMI (kg/m2) + 1.13 × IFG/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet count (×109 /L) − 0.66 × albumin (g/dL)
where IFG = impaired fasting glucose.
Interpretation
-
A score < −1.455 = lower risk of advanced fibrosis (F3/F4) in subjects > 65 years of age;
-
A score > 0.676 = high risk for advanced fibrosis (F3/F4) for all ages.
A close relationship between FIB-4 and NFS was described [24, 25].
Indeed, values of FIB-4 > 2.67 or NFS 0.676 identify people with a high probability of advanced liver fibrosis or advanced fibrosis (F3/F4), while people with FIB 0/1 or NFS < −1.455 are free of fibrosis, and those having FIB-4 Score F2 or NFS − 1.455 to 0.676 are undetermined [20, 21, respectively].
The cost for their calculation is negligible, and the result is immediate. Both scores were developed and validated for the identification of patients with NAFLD with a high probability of having bridging fibrosis (F3) or cirrhosis (F4) [24, 25, 30, 31].
Ultrasonography
Liver ultrasonography (US) was performed using the last generation Esaote MyLab™X75 equipped with SI2C41 convex ultrasound probe (Esaote S.p.A., Genoa, Italy), according to a validated procedure with a sensitivity of 100% and a specificity of 90% for the diagnosis of liver fat compared with liver biopsy in the event of ≥ 20% fatty liver (FL) content [27]. However, as precise FL quantification is still debated and awaiting validation on sufficiently large populations [27, 28], the US method served only as a screening tool at enrollment to assess the presence of FL, and a single US operator estimated FL content through FLI (with an inter-assay variability < 10%).
Treatment Adherence Assessment
At T-12 and T-24 visits, patients had to let the investigators analyze the drug boxes utilized during follow-up in search of any leftover sachets. Moreover, they all signed a questionnaire to record how often they inadvertently missed their expected dose. We considered “adherent” those taking at least 80% of the daily dosage [51]. To look for side effects, we also took advantage of a dedicated logbook consisting of ten dichotomic questions (yes/no answers) on gastrointestinal disorders.
Sample Size Calculation
We assumed that a 5.0% absolute difference in LF content between PGR and control groups would be the minimally appreciable and clinically relevant difference compared with baseline and the control group to have < 2.0% reduction in liver fat compared with the baseline, with a dropout rate of < 10%. With these assumptions, the sample size should be 100 per group to achieve a power of at least 90% with a p of 0.01. Therefore, we planned to randomize 125 participants per group to ensure adequate study power after any possible dropouts.
Statistical Procedures
Categorical data are presented as n (%). Continuous descriptive data were examined for normality, and normally distributed data were given as mean ± SD. Skewed data were presented as the median. To compare the two groups, the χ2 test or Fisher’s exact test were used for categorical variables, and the independent sample Student’s t test or Wilcoxon-Mann-Whitney U test were used to compare differences between continuous variables. The Pearson correlation coefficients (r) were determined by linear regression analysis. Univariate logistic regression was performed to screen for potential covariates associated with liver fat improvement. Covariates with p < 0.01 were entered into the multivariate model using the forward condition method. Multivariate linear regression analysis was conducted to estimate the effect of PGR on liver fat. Statistical analyses were performed by a biostatistician (MKS) using the SAS System version 9.4 (SAS Institute, Cary, NC, USA).
Results
Only two patients from the control group and three from the treatment group were excluded because of poor adherence to therapy. No one was excluded for side effects or adverse events, so 123 (CG) and 122 (TG) patients completed the study.
Table 1 displays general parameters, clearly showing superimposable values between groups. In particular, a typical MAFLD with high fatty liver content—as indicated by FLI—was observed in both the CG and the TG (94.31% vs. 95.08%, respectively), with AST and ALT γ-GT values higher than normal, as per enrollment criteria. Severe fibrosis was present in 88.25% vs. 83.60% of patients, respectively, as suggested by the FIB-3–4 Score, and medium fibrosis (i.e., F2) in 5.69% vs.7. 37%, respectively, with a normal condition or light fibrosis (F0–F1) in 9.75% vs. 9.01%, respectively. A similar trend was found at the NFS level for high and low fibrosis risk (i.e., 90.25% vs. 90.17% and 5.66% vs. 5.30%, respectively), with inconclusive results only observed at nominal rates (3.25% vs. 3.27%, respectively), with a 95.4% concordance rate between FIB and NFS scores. The chronic diabetes-related complications or comorbidities were similar, being hypertension 77.54% in the CG vs. 73.77% in the TG, cardio-vascular disease 62.82% vs. 61.56, and hypercholesterolemia 51.07% vs. 55.23%, respectively.
During the study, no participants had severe adverse effects, needed hospitalization, or died because of accidental causes or treatment. As reported in Table 2, gastrointestinal and general adverse effects were clinically irrelevant and comparable between groups. However, such symptoms caused no dropouts.
Table 3 synthesizes the least squares mean T24-T0 between-group treatment differences (– D %) in general parameters. The CG displayed no significant changes in any studied parameters, ranging from the lowest one, observed in HDL cholesterol (– 0.1 ± 0.4%) to the highest one, observed in HbA1c (– 3.5 ± 0.4%), with only a slight decrease in AST, ALT, γ-GT, and ALP. Instead, at the end of the follow-up, the TG displayed a significant reduction in AST, ALT, and γ-GT levels, and also in ALP, although less markedly so. In greater detail, AST reduced to normal in 12% vs. 79% of participants from the CG vs. those from the TG (p < 0.001), ALT did so in 9% vs. 80% (p < 0.001), respectively, and γ-GT in 9% vs. 78% (p < 0.001), respectively (see Fig. 3).
In greater detail, no significant differences were observed between groups during the time interval T0–T24 (Δ%) for albumin (± 0.2 ± 0.04% in the CG vs. ± 0.2 ± 0.04% int the TG), platelet count (– 0.9 ± 0.1% vs. – 0.8 ± 0.1%, respectively), and eGFR (– 0.4 ± 0.1% vs. – 0.6 ± 0.1%, respectively), while all other parameters improved significantly in the TG vs. the CG, including BMI (kg/m2) (– 1.2 ± 0. 01 vs. – 4.8 ± 0.4%, CI 2.201–6.466, p < 0.01), HbA1c (%) (– 3.5 ± 0. 4 vs. – 8.2 ± 1.0%, CI 0.686–3.318, p < 0.01), FPG (mg/dl) (– 1.6 ± 0.9 vs. – 7.7 ± 0.8%, CI 0.637–4.031, p < 0.01), PPG (postprandial glycemia; mg/dl) (– 2.5 ± 1.8 vs. – 8.6 ± 2.2%, CI 1.184–6.981, p < 0.001), total cholesterol (mg/dl) (– 2.5 ± 1.0 vs. – 9.9 ± 1.1%, CI 0.682–5.919, p < 0.001), LDL cholesterol (mg/dl) (– 2.4 ± 0.6 vs. – 9.9 ± 1.2%, CI 0.910–5.128, p < 0.01), and triglycerides (mg/dl) (– 2.5 ± 1.1 vs. – 9.8 ± 0.6%, CI 0.912–5.112, p < 0.01). A marked and significant variation between CG and TG during the T0–T24 period was observed in free insulin (μIU/ml) (– 1.3 ± 0.5% vs. – 38.6 ± 0.6%, respectively; CI 0.993–4.756, p < 0.0001), HOMA-IR (– 5.2 ± 0.4% vs. – 42.2 ± 0.6%, respectively; CI 0.789–4.235, p < 0.0001), and, less prominently, C-peptide (ng/ml) (– 0.4 ± 0.1% vs. – 7.2 ± 0.4%, respectively, CI 0.346–1.878, p < 0.05).
T0-T24 variations in body fat are presented in Fig. 2, including waist circumference (WC), trunk fat (TF), and visceral fat (VF). Both CG and TG displayed a significant decrease in all three parameters, despite an almost double effect being observed in the TG, with the highest variation occurring in VF (– 11.9 ± 4.3% vs. – 25.5.6%, CI 2.130–7.543, p < 0.001, respectively) (Table 4). Complication rates and comorbidities were similar between groups (data not reported in the Table 3).
Figure 3 displays FLI distribution in the two groups at baseline and end of follow-up. T0–T24 changes were virtually null in the CG (ranging from 0 to 3.69%; see Fig. 3). Contrarily, in the TG, the prevalence of subjects with severe steatosis (FLI > 60) significantly decreased from 95.08 to 47.53% (p < 0.001), with a concomitant significant increase in that of subjects with FLI < 30 from 1.63 to 49.3% (p < 0.001), with virtually no change in the prevalence of inconclusive results.
Comparison of the percentages of subjects with (FLI < 30), without (FLI > 60), and undetermined (FLI between 31 and 59) NAFLD at baseline and the end of follow-up: *p < 0.001 vs. baseline; #p < 0.001 vs. the other group. FLI Fatty Liver Index, NFS NAFLD (non-alcoholic fatty liver disease) Fibrosis Score
When considering the FIB-4 (Fig. 4), at baseline medium-severe degrees (F3–F4) were similar in both groups (84.56% in the CG vs. 83.62% in the TG, p = ns). The same applied to low (F0–F1) (9.75% vs. 9.01, respectively, p = ns) and moderate degrees (5.69% vs. 7.37%, respectively, p = ns).
Comparison of FIB-4 in subjects without or with light fibrosis (FIB-4 < 1.30; F0-F1), severe fibrosis (FIB-4 > 2.67; F3-F4), and moderate diagnosis (FIB-4 between 1.31 and 2.66; F2) between baseline and end of follow-up. **p < 0.001 vs. baseline and CG. FIB-4 Fibrosis (FIB)-4 Score, CG control group
T0-T24 variations after treatment were virtually null in the CG, while the prevalence of medium-severe cases (F3-F4) decreased significantly by 83.62% to 52.35% (p < 0.001) in the TG; that of low degrees (F0–F1) markedly increased by 9.01–42.15% (p < 0.001).
Figure 5 shows NFS results, which were virtually superimposable to this from FIB-4, with a maximal 95% concordance and a very low prevalence of inconclusive scores in both groups throughout the T0–T24 period. The prevalence of extreme scores stayed almost unchanged only in the CG, while improving significantly in the TG both within and between groups (p < 0.001): high probability scores (NFS > 0.6) decreased by 90.13% to 59.10%, p < 0.001), low probability scores (NFS < −1.455) increased by 6.65–37.80%, p < 0.001), while inconclusive cases were similar (3.27% vs 3.19%, respectively).
Comparison of percentages of subjects with (NFS > 0.676) and without (NFS < −1.455) advanced liver fibrosis, or inconclusive results (NFS −1.455 to 0.676), between baseline and the end of follow-up; *p < 0.001 vs. baseline; #p < 0.001 vs. the other group. NFS NAFLD (non-alcoholic fatty liver disease) Fibrosis Score
Finally, Fig. 6 displays the significantly different changes occurring in ASL, ALT, and γ-GT in the PGR compared to the CG (p < 0.01).
Mean values and SDs of AST (IU/l), ALT (IU/l), and γ-GT (GGT; IU/l) in the two groups at baseline and end follow-up and significance of differences. *p < 0.01 and **p < 0.05 vs. control group: °p < 0.01 and °°p < 0.05 vs. baseline. ALT alanine transferase, AST aspartate transferase, γ-GT gamma-glutamyl transferase
Discussion
The liver is the largest metabolic organ of the body and, as such, integrates numerous metabolic processes, including lipid and glucose metabolism and xenobiotic detoxification. Several hepatic metabolic pathways follow circadian patterns and are highly susceptible to nutrient metabolism challenges so that fatty liver results in disrupted circadian metabolic rhythms, insulin resistance, and consequent development of obesity and Type 2 diabetes, largely involved in the clinical phenotype of metabolic syndrome [35, 52,53,54].
NAFLD affects about 25% of the adult population [1]. Its prevalence increases in parallel to that of obesity and MS, with which it is intimately associated [1], even raising questions about its nosographic definition and changing it into MAFLD, where “M” stay for “metabolic,” indicating association with a long-standing, clinically evident diabetic condition [1, 2, 5, 6].
Indeed, NAFLD pathophysiology relies on factors contributing to the MS, including weight gain/obesity, insulin resistance, visceral obesity, dyslipidemia, hypertension, and prediabetes or clinically evident diabetes (in which case the name changes into MAFLD; 40, 41), besides physical inactivity, chronic inflammatory conditions, and altered gut microbiota. Clinicians have been looking for a single treatment tool against it for years without success. However, recent studies reported a series of metabolic benefits as a function of treatment with macromolecular complex based on polysaccharides and covered by a European patent (no. 1679009), slowing the rate of carbohydrate and fat absorption (Neo-Policaptil Gel Retard®). It is derived from high-fiber raw materials including glucomannan (Amorphophallus konjac), cellulose Opuntia pulp stem Opuntia ficus indica), chicory root (Cichorium intybus), freeze-dried mallow root mucilage (Althaea officinalis), freeze-dried flaxseed mucilage (Linum usitatissimum), and freeze-dried linden flower mucilage (Tilia platyphyllos Scop), as previously described [32].
PGR significantly reduces acanthosis nigricans expressions, HbA1c levels, and glucose metabolism abnormalities, including impaired glucose tolerance (IGT) and T2DM, in hyper-insulemic adolescents [32], because of blunted post-meal blood glucose and insulin peaks [33, 34, 42]. The attenuated insulin response to the PGR’s regulating and delaying activity on gastrointestinal glucose absorption [58, 59] decreases body weight, visceral fat, and cardiovascular risk [42,43,44].
Moreover, in the animal model, reduced carbohydrate and lipid availability to gut epithelium positively affects microbiota by favoring the growth of the Bacteroides phylum, endowed with anti-lipid absorption properties, against the previously overexpressed Firmicutes phylum, notoriously responsible for increased lipid gut absorption [56]. PGR also completely blocks the induction of genes involved in fat accumulation in the liver of mice treated with a high-fat diet (HFD) to reproduce a model of human MS during the night, thus positively modulating lipid metabolism, including liver cholesterol and fatty acid storage and triglyceride storage-associated protein (CD36) [56].
Our data provide support to the favorable effects of PGR plus a slightly hypocaloric diet on body weight and fat distribution, particularly evident in terms of visceral fat, as documented by the almost double VF reduction observed in the TG compared to the CG along with a significant decrease in plasma lipids and high tolerability.
However, the main result of our study was the 70.76% lower liver fat content attained by the TG compared to the only 2.5% change of the CG (p < 0.001), showing that only small hepatic changes can be obtained through diet, while PGR is much more effective.
The special feature of our study is the relevantly better fibrosis markers FIB-4 and NFS observed in treated subjects with a 95% concordance. PGR improved fibrosis extent significantly not only in those with high scores (FIB-4 F3–F4 and NFS > 0.626) but also in less severe cases (F0–F2 and < −1.455, respectively). Such results are quite new and difficult to explain without considering PGR’s ability to hinder glucotoxicity and lipotoxicity in adolescents and adults with MS [32, 33, 42, 44] and to improve the microbiota [42, 46, 47].
Based on animal studies where gut microbiota was manipulated and observational studies on human NAFLD, considerable evidence has emerged concerning the ability of altered microbiota to cause NAFLD through increased gut permeability to bacterial products and consequent liver inflammation and fibrosis triggered by harmful debris. Dysbiosis, combined with poor lifestyle and diet habits, also changes the luminal metabolism of food substrates through increased production of certain short-chain fatty acids and alcohol and depletion of choline. When such local environmental changes occur, patients experience gut inflammation, altered motility, and immune function, eventually leading to liver damage. However, gut microbiota analysis is expected to improve, thus allowing clinicians to identify appropriate treatment tools against NAFLD and associated disorders through a better understanding of subtle interactions among dysbiosis, diet, and environmental factors [42, 46, 47].
Finally, for the first time, we documented significantly improved AST, ALT, and γ-GT patterns in adults with MAFLD treated with PGR, opposite to those on a diet only. A possible explanation for this is the reduced liver fat content and consequently feer apoptotic changes dependent on excess fat, less relevant insulin resistance, and lower oxidative stress due to gluco-/lipo-toxicity and microbiota-related chronic low-grade inflammation.
Limitations
Despite several therapeutic attempts against NAFLD/NASH, long-term results were inconclusive in most cases [48]. In particular, a relatively short period spent on a Mediterranean diet associated with increased physical activity proved effective against NAFLD/NASH. However, long-term evidence of effectiveness is lacking in this case because of the well-known phenomenon of progressive adherence decay that all too often afflicts every lifestyle change attempt [49]. Our study has the intrinsic limitation of evaluating liver fat and fibrosis only through validated yet indirect indices, including FLI, FIB-4, and NFS. Therefore, further morphology-based studies, possibly associated with gut microbiota analysis, are needed to confirm our results. However, it has the merit of proposing for the first time a single, easy-to-handle treatment tool acting on different targets simultaneously, including metabolic and inexpensive liver morphology-related parameters that are readily available in large outpatient cohorts and thus allow our investigation to be easily repeated worldwide.
We did not use a comparator for the following reasons:
-
The study was not expected to assess the superiority or non-inferiority of PGR on another molecule.
-
Previous studies from other groups and ours have already shown that PGR significantly modifies the metabolic parameters involved in liver fat [34, 35, 57, 60, 71]; what had not been investigated and documented so far were the effects of PGR on the amount of stored liver fat and degree of fibrosis. Indeed, although we already showed the PGR's non-inferiority to metformin concerning glucose control and superiority in terms of lipids and body fat following a similar protocol [34], the aims of the present article were totally different; therefore, we considered duplicating a head-to-head comparison with the other molecule redundant.
Conclusions
NAFLD is widespread worldwide and is present in over half of T2DM subjects; it simultaneously increases the risk of T2DM in non-diabetic subjects, just as T2DM increases the risk of NAFLD [47]. Moreover, about one-third of people with NAFLD progress to NASH, thus running an increased risk of cirrhosis or even hepatocellular carcinoma. Nevertheless, the inner mechanisms behind NAFLD are still a matter of debate, and the same applies to standard NAFLD/NASH diagnosis and treatment criteria [46, 47].
In people with T2DM, NASH causes impaired hepatic function through the aggravation of pre-existing chronic low-grade inflammation [50,51,52] and the structural changes caused by increased fat stores. This phenomenon, in turn, reduces efficient and safe hypoglycemic treatment options for those patients, although some experimental studies look promising regarding some—still somewhat expensive—new entries in the therapeutic toolkit. Indeed, sodium-glucose cotransporter-2 inhibitors (SGLT-2is) [52] and the dipeptidyl peptidase-4 inhibitor (DPP-4i) sitagliptin [54] proved beneficial for NAFLD/NASH in mouse models and humans, respectively. In addition, previous preliminary experiences pointed to a possible positive impact of glucagon-like peptide-1 receptor agonists (GLP-1RAs) on lipid oxidation and hepatic signaling alterations induced by a high-fat diet in non-alcoholic steatohepatitis [55, 56]. In greater detail, several old and new pharmacologic approaches approved for T2DM treatment were used in people with T2DM and NAFLD, including metformin, thiazolidinediones, insulin sensitizers, GLP-1Ras (exenatide, liraglutide, dulaglutide, and semaglutide), and DPP-4is approved, while SGLT-2is, nuclear receptor modulators, PPAR agonists, thyroid hormone receptor agonists (THRβAs), inhibitors of de novo lipogenesis (DNL), ketohexokinase inhibitors, and fibroblast growth factor regulators were tested in different experimental conditions [57, 58]. However, the efficacy and durability of all such attempts are still debated and await further investigation.
In addition, we found it very interesting that LFI, FIB-4, and NFS, i.e., easy, non-invasive, and low-cost tests validated in different populations and extensively used in the literature, especially for screening purposes, may represent a valuable diagnostic tool for any clinicians interested in the field [21, 31, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77].
Finally, our data show for the first time that PGR, in addition to lifestyle changes including a low-calorie diet and intensified physical activity in adults affected by metabolic syndrome and type 2 diabetes with NAFLD, nominally MAFLD, improves lipid and glucose metabolism-related parameters, including insulin resistance, and significantly reduces not only visceral fat but also liver fat content and related liver fibrosis severity. We also emphasize that, although we already found PGR to have a positive effect on metabolic parameters, lipids, and fat redistribution [35] and a significantly improved individual 10-year cardiovascular risk, even more than metformin [44], through this study, we showed for the first time that PGR significantly improves liver lipid content and fibrous components.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Change history
22 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s13300-024-01553-2
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. https://doi.org/10.1002/hep.28431. (PMID: 26707365).
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85.
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. https://doi.org/10.1016/j.jhep.2015.11.004.
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–9. https://doi.org/10.1016/j.jhep.2020.03.039. (PMID: 32278004).
Eslam M, Sanyal AJ, George J, International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999-2014.e1. https://doi.org/10.1053/j.gastro.2019.11.312. (PMID: 32044314).
Kleiner DE, Brunt EM, Wilson LA, et al. Nonalcoholic steatohepatitis clinical research network. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open. 2019;2(10): e1912565. https://doi.org/10.1001/jamanetworkopen.2019.12565. (PMID: 31584681; PMCID: PMC6784786).
De A, Duseja A. Natural history of simple steatosis or nonalcoholic fatty liver. J Clin Exp Hepatol. 2020;10(3):255–62. https://doi.org/10.1016/j.jceh.2019.09.005.PMID:32405182;PMCID:PMC7212297.
McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–55. https://doi.org/10.1016/j.jhep.2014.11.034. (PMID: 25477264).
Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643-54.e9. https://doi.org/10.1016/j.cgh.2014.04.014. (PMID: 24768810; PMCID: PMC4208976).
Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600.
Kleiner DE, Brunt EM, Van Natta M, et al. Nonalcoholic steatohepatitis clinical research network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. https://doi.org/10.1002/hep.20701. (PMID: 15915461).
Kawada N, Imanaka K, Kawaguchi T, et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(12):1190–4. https://doi.org/10.1007/s00535-009-0112-0. (PMID: 19672551).
Younossi Z, Stepanova M, Ong JP, et al. Global nonalcoholic steatohepatitis council. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–7553. https://doi.org/10.1016/j.cgh.2018.05.057. (PMID: 29908364).
Musso G, Gambino R, Tabibian JH, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7): e1001680. https://doi.org/10.1371/journal.pmed.1001680.PMID:25050550;PMCID:PMC4106719.
Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–65. https://doi.org/10.1002/hep.29085. (PMID: 28130788; PMCID: PMC5397356).
Mouries J, Brescia P, Silvestri A, et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71(6):1216–28. https://doi.org/10.1016/j.jhep.2019.08.005. (PMID: 31419514; PMCID: PMC6880766).
Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-97.e10. https://doi.org/10.1053/j.gastro.2015.04.043. (PMID: 25935633; PMCID: PMC4516664).
Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–73. https://doi.org/10.1016/j.jhep.2017.07.027. (PMID: 28803953).
Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1611-1625.e12. https://doi.org/10.1053/j.gastro.2020.01.043. (PMID: 32027911).
Yamamichi N, Shimamoto T, Okushin K, et al. Fibrosis-4 index efficiently predicts chronic hepatitis and liver cirrhosis development based on a large-scale data of general population in Japan. Sci Rep. 2022;12(1):20357. https://doi.org/10.1038/s41598-022-24910-2. (PMID: 36437271; PMCID: PMC9701772).
McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems canreliably exclude advanced fibrosis in patientswith non-alcoholic fatty liver disease. Gut. 2010;59:1265–9.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–57. https://doi.org/10.1002/hep.29367. (PMID: 28714183).
Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–6. https://doi.org/10.1002/hep.21669. (PMID: 17567829).
Mózes FE, Lee JA, Selvaraj EA, LITMUS Investigators, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2022;71(5):1006–19. https://doi.org/10.1136/gutjnl-2021-324243. (PMID: 34001645; PMCID: PMC8995830).
Treeprasertsuk S, Björnsson E, Enders F, Suwanwalaikorn S, Lindor KD. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroenterol. 2013;19(8):1219–29. https://doi.org/10.3748/wjg.v19.i8.1219.PMID:23482703;PMCID:PMC3587478.
Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1851–64. https://doi.org/10.1053/j.gastro.2020.01.052. (PMID: 32061595).
Ballestri S, Nascimbeni F, Lugari S, Lonardo A, Francica G. A critical appraisal of the use of ultrasound in hepatic steatosis. Expert Rev Gastroenterol Hepatol. 2019;13(7):667–81. https://doi.org/10.1080/17474124.2019.1621164. (PMID: 31104523).
Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25(40):6053–62. https://doi.org/10.3748/wjg.v25.i40.6053.PMID:31686762;PMCID:PMC6824276.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;2(6):33. https://doi.org/10.1186/1471-230X-6-33.PMID:17081293;PMCID:PMC1636651.
Siddiqui MS, Yamada G, Vuppalanchi R, et al. NASH clinical research network. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. 2019;17(9):1877-1885.e5. https://doi.org/10.1016/j.cgh.2018.12.031. (PMID: 30616027; PMCID: PMC6609497).
Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–54. https://doi.org/10.1002/hep.21496. (PMID: 17393509).
Guarino G, Della Corte T, Strollo F, Gentile S. Policaptil gel retard in adult subjects with the metabolic syndrome: efficacy, safety, and tolerability compared to metformin. Diabetes Metab Syndr. 2021;15(3):901–7. https://doi.org/10.1016/j.dsx.2021.03.032. (PMID: 33906073).
Stagi S, Lapi E, Seminara S, et al. Policaptil Gel Retard significantly reduces body mass index and hyperinsulinism and may decrease the risk of type 2 diabetes mellitus (T2DM) in obese children and adolescents with family history of obesity and T2DM. Ital J Pediatr. 2015;15(41):10. https://doi.org/10.1186/s13052-015-0109-7. (PMID: 25774705; PMCID: PMC4339082).
Stagi S, Ricci F, Bianconi M, et al. Retrospective evaluation of metformin and/or metformin plus a new polysaccharide complex in treating severe hyperinsulinism and insulin resistance in obese children and adolescents with metabolic syndrome. Nutrients. 2017;9(5):524. https://doi.org/10.3390/nu9050524. (PMID: 28531113; PMCID: PMC5452254).
American Diabetes Association Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–38. https://doi.org/10.2337/dc22-S002. (PMID: 34964875).
Shield KD, Gmel G, Gmel G, et al. Life-time risk of mortality due to different levels of alcohol consumption in seven European countries: implications for low-risk drinking guidelines. Addiction. 2017;112(9):1535–44. https://doi.org/10.1111/add.13827. (PMID: 28318072).
Arab JP, Roblero JP, Altamirano J, et al. Alcohol-related liver disease: clinical practice guidelines by the Latin American association for the study of the liver (ALEH). Ann Hepatol. 2019;18(3):518–35. https://doi.org/10.1016/j.aohep.2019.04.005. (PMID: 31053546).
Devarbhavi H, Aithal G, Treeprasertsuk S, et al. Drug-induced liver injury: Asia pacific association of study of liver consensus guidelines. Hepatol Int. 2021;15:258–82. https://doi.org/10.1007/s12072-021-10144-3.
Baranauskas MN, Johnson KE, Juvancic-Heltzel JA, Kappler RM, Richardson L, Jamieson S, Otterstetter R. Seven-site versus three-site method of body composition using BodyMetrix ultrasound compared to dual-energy X-ray absorptiometry. Clin Physiol Funct Imag. 2017;37(3):317e21. https://doi.org/10.1111/cpf.12307.
Alomari M, Rashid MU, Chadalavada P, et al. Comparison between metabolic-associated fatty liver disease and nonalcoholic fatty liver disease: from nomenclature to clinical outcomes. World J Hepatol. 2023;15(4):477–96. https://doi.org/10.4254/wjh.v15.i4.477. (PMID: 37206648; PMCID: PMC10190689).
Greco CM, Garetto S, Montellier E, et al. A non-pharmacological therapeutic approach in the gut triggers distal metabolic rewiring capable of ameliorating diet-induced dysfunctions encompassed by metabolic syndrome. Sci Rep. 2020;10(1):12915. https://doi.org/10.1038/s41598-020-69469-y. (PMID: 32737396; PMCID: PMC7395094).
Fornari E, Morandi A, Piona C, Tommasi M, Corradi M, Maffeis C. Policaptil gel retard intake reduces postprandial triglycerides, ghrelin and appetite in obese children: a clinical trial. Nutrients. 2020;12(1):214. https://doi.org/10.3390/nu12010214. (PMID: 31947628; PMCID: PMC7019363).
Guarino G, Della Corte T, Strollo F, et al. Comparison between policaptil gel retard and metformin by testing of temporal changes in patients with metabolic syndrome and type 2 diabetes. Diabetology. 2022;3:315–27. https://doi.org/10.3390/diabetology3020022.
Guarino G, Strollo F, Malfertheiner P, et al. Efficacy and safety of a polysaccharide-based natural substance complex in the treatment of obesity and other metabolic syndrome components: a systematic review. Front Drug Saf Regul. 2022;2: 844256. https://doi.org/10.3389/fdsfr.2022.844252222.
Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–25. https://doi.org/10.1038/nrgastro.2016.85. (PMID: 27273168).
Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 2019;1:312–8. https://doi.org/10.1016/j.jhepr.2019.07.002.
Tanase DM, Gosav EM, Costea CF, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res. 2020;2020:3920196. https://doi.org/10.1155/2020/3920196.
Rong L, Zou J, Ran W, Qi X, Chen Y, Cui H, Guo J. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front Endocrinol (Lausanne). 2023;16(13):1087260. https://doi.org/10.3389/fendo.2022.1087260. (PMID: 36726464; PMCID: PMC9884828).
Montemayor S, Mascaró CM, Ugarriza L, Casares M, Llompart I, Abete I, Zulet MÁ, Martínez JA, Tur JA, Bouzas C. Adherence to Mediterranean diet and NAFLD in patients with metabolic syndrome: the FLIPAN study. Nutrients. 2022;14(15):3186. https://doi.org/10.3390/nu14153186. (PMID: 35956364; PMCID: PMC9370227).
Colak E, Pap D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J Med Biochem. 2021;40:1–9. https://doi.org/10.5937/jomb0-24652.
Saxena NK, Titus MA, Ding X, et al. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J. 2004;18:1612–4. https://doi.org/10.1096/fj.04-1847fje.
Musso G, Gambino R, Pacini G, Pagano G, Durazzo M, Cassader M. Transcription factor 7-like 2 polymorphism modulates glucose and lipid homeostasis, adipokine profile, and hepatocyte apoptosis in NASH. Hepatology. 2009;49:426–35. https://doi.org/10.1002/hep.22659.
Yabiku K. Efficacy of sodium-glucose cotransporter 2 inhibitors in patients with concurrent type 2 diabetes mellitus and non-alcoholic steatohepatitis: a review of the evidence. Front Endocrinol. 2021;12: 768850. https://doi.org/10.3389/fendo.2021.768850.
Cui J, Philo L, Nguyen P, et al. Sitagliptin vs placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016;65(2):369–76. https://doi.org/10.1016/j.jhep.2016.04.021. (PMID: 27151177; PMCID: PMC5081213).
Kuchay MS, Krishan S, Mishra SK, et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial). Diabetologia. 2020;63(11):2434–45. https://doi.org/10.1007/s00125-020-05265-7. (PMID: 32865597).
Gentile S, Strollo G, Guarino G, et al. Effect of dulaglutide treatment in aged subjects with type 2 diabetes mellitus and fatty liver disease. J Diab Metab Disorder. 2023;10(1):56–64. https://doi.org/10.15406/jdmdc.2023.10.00253.
Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):484–95. https://doi.org/10.1038/s41574-021-00507-z. (PMID: 34131333; PMCID: PMC8570106).
Raza S, Rajak S, Upadhyay A, Tewari A, Anthony SR. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci (Landmark Ed). 2021;26(2):206–37. https://doi.org/10.2741/4892. (PMID: 33049668; PMCID: PMC7116261).
Motamed N, Faraji AH, Khonsari MR, et al. Fatty liver index (FLI) and prediction of new cases of non-alcoholic fatty liver disease: a population-based study of northern Iran. Clin Nutr. 2020;39(2):468–74. https://doi.org/10.1016/j.clnu.2019.02.024. (PMID: 30922791).
de Lédinghen V, Vergniol J, Barthe C, et al. Non-invasive tests for fibrosis and liver stiffness predict 5-year survival of patients chronically infected with hepatitis B virus. Aliment Pharmacol Ther. 2013;37(10):979–88. https://doi.org/10.1111/apt.12307.
Younossi ZM, Noureddin M, Bernstein D, et al. Role of noninvasive tests in clinical gastroenterology practices to identify patients with nonalcoholic steatohepatitis at high risk of adverse outcomes: expert panel recommendations. Am J Gastroenterol. 2021;116(2):254–62. https://doi.org/10.14309/ajg.0000000000001054.
Wu S, Yang Z, Zhou J, et al. Systematic review: diagnostic accuracy of non-invasive tests for staging liver fibrosis in autoimmune hepatitis. Hepatol Int. 2019;13(1):91–101. https://doi.org/10.1007/s12072-018-9907-5.
Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–25. https://doi.org/10.1002/hep.21178.
Sun W, Cui H, Li N, et al. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: a meta-analysis study. Hepatol Res. 2016;46(9):862–70. https://doi.org/10.1111/hepr.12647.
Demir M, Lang S, Nierhoff D, Drebber U, Hardt A, Wedemeyer I, Schulte S, Quasdorff M, Goeser T, Töx U, Steffen HM. Stepwise combination of simple noninvasive fibrosis scoring systems increases diagnostic accuracy in nonalcoholic fatty liver disease. J Clin Gastroenterol. 2013;47(8):719–26. https://doi.org/10.1097/MCG.0b013e3182819a89. (PMID: 23442837).
Wong VW, Wong GL, Chim AM, et al. Validation of the NAFLD fibrosis score in a Chinese population with low prevalence of advanced fibrosis. Am J Gastroenterol. 2008;103(7):1682–8. https://doi.org/10.1111/j.1572-0241.2008.01933.x. (PMID: 18616651).
EASL-ALEH Clinical Practice Guidelines. Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–64. https://doi.org/10.1016/j.jhep.2015.04.006. (View with CPSBC or UBC).
Kumar R, Teo EK, How CH, Wong TY, Ang TL. A practical clinical approach to liver fibrosis. Singap Med J. 2018;59(12):628–33. https://doi.org/10.11622/smedj.2018145. (PMID: 30631885; PMCID: PMC6301869).
Reinshagen M, Kabisch S, Pfeiffer AFH, Spranger J. Liver fat scores for noninvasive diagnosis and monitoring of nonalcoholic fatty liver disease in epidemiological and clinical studies. J Clin Transl Hepatol. 2023;11(5):1212–27. https://doi.org/10.14218/JCTH.2022.00019. (PMID: 37577225; PMCID: PMC10412706).
Zhang F, Han Y, Zheng L, et al. Association of non-invasive markers with significant fibrosis in patients with nonalcoholic fatty liver disease: a cross-sectional study. Diabetes Metab Syndr Obes. 2023;31(16):2255–68. https://doi.org/10.2147/DMSO.S417754. (PMID: 37545743; PMCID: PMC10403050).
Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441–7. https://doi.org/10.1136/gut.2007.146019. (PMID: 18390575).
Han S, Choi M, Lee B, et al. Accuracy of noninvasive scoring systems in assessing liver fibrosis in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gut Liver. 2022;16(6):952–63. https://doi.org/10.5009/gnl210391. (PMID: 35193993; PMCID: PMC9668505).
Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486–501. https://doi.org/10.1002/hep.29302. (PMID: 28586172).
Jaruvongvanich V, Wijarnpreecha K, Ungprasert P. The utility of NAFLD fibrosis score for prediction of mortality among patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis of cohort study. Clin Res Hepatol Gastroenterol. 2017;41(6):629–34. https://doi.org/10.1016/j.clinre.2017.03.010. (PMID: 28716600).
Rigor J, Diegues A, Presa J, Barata P, Martins-Mendes D. Noninvasive fibrosis tools in NAFLD: validation of APRI, BARD, FIB-4, NAFLD fibrosis score, and Hepamet fibrosis score in a Portuguese population. Postgrad Med. 2022;134(4):435–40. https://doi.org/10.1080/00325481.2022.2058285. (PMID: 35332833).
Lee J, Vali Y, Boursier J, Spijker R, Anstee QM, Bossuyt PM, Zafarmand MH. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: a systematic review. Liver Int. 2021;41(2):261–70. https://doi.org/10.1111/liv.14669. (PMID: 32946642; PMCID: PMC7898346).
Lombardi R, Airaghi L, Targher G, et al. NAFLD fibrosis score (NFS) can be used in outpatient services to identify chronic vascular complications besides advanced liver fibrosis in type 2 diabetes. J Diabetes Complicat. 2020;34(11): 107684.
Acknowledgements
We are indebted to Dr. Paola Murano, General Manager of the Nefrocenter Research Network, for the effective and continuous support offered complimentarily for the realization of the manuscript. Special thanks are due to La. Fa. Re. Laboratorio Farmaceutico Reggiano, Via Sacerdote Benedetto Cozzolino, 77 Ercolano (NA), for preparing the placebo for free. Special thanks are due to the patients enrolled in the study.
Funding
No funding or sponsorship was received for this study or the publication of this article. None of the authors or coworkers received funding or another type of payment for this paper.
Author information
Authors and Affiliations
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article taking responsibility for the integrity of the work as a whole and gave their approval for this version to be published. Sandro Gentile and Felice Strollo designed the study and wrote the article. Giuseppina Guarino, Ersilia Satta, and Teresa Della Cotre critically read and approved the paper. All authors contributed to data acquisition, critically assessed the results, and approved the final text.
Corresponding author
Ethics declarations
Conflict of Interest
Sandro Gentile, Felice Strollo, Giuseppina Guarino, Ersilia Satta, and Teresa Della Corte have no financial interests to declare in relation to the present study.
Ethical Approval
The Ethics Committee of the University Hospital of Campania "Luigi Vanvitelli," Naples, Italy, approved the present study (protocol no. 33478/2022, November 11, 2022). All participants signed informed consent, and data were processed anonymously according to good clinical practice guidelines. This study was conducted in conformance with good clinical practice standards. The study was led in accordance with the Declaration of Helsinki 1975, as subsequent amendments. All followed procedures were in accordance with the ethical standards of the responsible committee on human experimentation (both institutional and national) and with usual clinical practice. Written informed consent was obtained from all participants before enrollment.
Additional information
This article was revised due to update in Figures 3, 4, 5 & 6. There were few corrections within the text.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Guarino, G., Strollo, F., Della Corte, T. et al. Effect of Neo-Policaptil Gel Retard on Liver Fat Content and Fibrosis in Adults with Metabolic Syndrome and Type 2 Diabetes: A Non-invasive Approach to MAFLD. Diabetes Ther 14, 2089–2108 (2023). https://doi.org/10.1007/s13300-023-01478-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01478-2