Abstract
Introduction
Hemoglobin A1c (HbA1c), representing the average blood glucose over 1–2 months, is the most commonly used glycemic marker in people with diabetes. Glycated albumin (GA) reflects the average blood glucose over the most recent 1–2 weeks. We considered whether the faster response of GA compared with HbA1c could make people with diabetes realize their glycemic control intuitively and effectively.
Methods
We randomized 61 people with diabetes into the control and intervention groups. Blood samples were collected from both every fortnight over an 8-week period (five times; visit 1–5). Only the intervention group was notified of the GA levels on the same day. At the beginning and end of the study, International Physical Activity Questionnaire and Eating Behavior Questionnaire assessments, and body composition measurements were conducted.
Results
The body weight change was significantly lower in the intervention group at visit 2 and visit 5. The percent body fat change was lower, while the percent skeletal muscle mass change at visit 5 was higher in the intervention group. Increasing GA trend was observed in the control group, but not in the intervention group. The fasting plasma glucose and HbA1c changes at visit 5 were similar in the two groups. Physical activity level change tended to be higher in the intervention group. The YN Eating Behavior Questionnaire score changes were similar in the two groups.
Conclusion
Bi-weekly GA measurement over an 8-week period in people with type 2 diabetes induced behavioral changes. Development of this method is expected to improve diabetes management.
Trial Registration
UMIN000037795.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Hemoglobin A1c (HbA1c) reflects the average blood glucose level over the previous 1–2 months, while the glycated albumin (GA) reflects the average blood glucose level over the most recent 1–2 weeks. |
The faster response of GA as compared with HbA1c can lead to more effective glycemic control in people with diabetes. |
What was learned from the study? |
Bi-weekly GA measurement over an 8-week period in people with type 2 diabetes significantly improved the body weight and body composition. |
The eating behavior improved with the intervention. |
Routine GA measurement might improve obesity-related conditions such as type 2 diabetes. |
Introduction
Intensive glycemic control reduces the risk of micro- and macrovascular complications [1,2,3], cancer [4, 5], and dementia [6] in people with type 2 diabetes mellitus. Reduction of these complications could decrease the mortality of people with diabetes and improve their quality of life. While the level of hemoglobin A1c (HbA1c) is the most commonly used marker of the glycemic status in people with diabetes, it reflects the average blood glucose level over the previous 1–2 months. The relatively slow response of this parameter to blood glucose changes makes it unsuitable for accurately monitoring the glycemic control status. Hence, better tools for blood glucose monitoring have been sought.
Recently, attention has been focused on self-monitoring of blood glucose (SMBG) or continuous glucose monitoring (CGM). While these tools have been reported to provide valuable information of the glycemic status and improve glycemic control [7,8,9], they are not necessarily suitable for all patients with diabetes. Improvement of glycemic control with SMBG has been reported to be small in people with type 2 diabetes who do not use insulin [10], despite the need for frequent finger pricks. In the case of CGM, the need for placement of a sensor under the skin adds to the burden of people with diabetes. Further, interpretation of the results of CGM is also not straightforward [11, 12]. Thus, no optimal method for monitoring blood glucose has been established yet [13, 14].
We therefore focused our attention on glycated albumin (GA). The GA level reflects the average blood glucose levels over the most recent 1–2 weeks [15]. The faster response of GA as compared with HbA1c to changes of the blood glucose level is derived from the shorter half-life of serum albumin (14–20 days) [16,17,18], as compared with that of hemoglobin (about 120 days) [19, 20]. Thus, GA measurement may enable more accurate monitoring of the glycemic status than HbA1c. Furthermore, it might also be a less invasive, less burdensome, and more economical tool for blood glucose monitoring than SMBG or CGM.
To eventually realize more effective glycemic control in people with diabetes intuitively, in this study we examined whether periodic GA monitoring might encourage behavioral changes and improve the blood glucose levels in people with diabetes. We measured the blood GA levels every 2 weeks and notified the result of the measurement to each study participant on the same day as the blood sample collection. Behavioral changes were evaluated using questionnaires, in addition to measurement of the body weight, body composition, and biochemical parameters, considering that dyslipidemia [21] and liver dysfunction [22] are commonly associated with diabetes. Moreover, this study was conducted in autumn, when the HbA1c and cholesterol levels are generally relatively elevated in Japanese people with diabetes [23, 24].
Methods
This single-center, open-label, randomized, parallel-group study was conducted to examine the effect of notifying participants with diabetes of their GA levels in improving the glycemic control in these participants. The study protocol was approved by the Research Ethics Committee of the University of Tokyo Hospital (2019092NI), and the study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent to this study was obtained from each of the participants after an explanation about the study was provided.
Participants
Participants with type 2 diabetes mellitus aged at least 20 years old were recruited for this study. The exclusion criteria, overall, included carriers of syphilis, hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, HbA1c values of less than 6.0% or 9.0% and higher, and/or history of receiving insulin or GLP-1 receptor agonist injections for the treatment of diabetes mellitus. Participants were randomly assigned to two groups, the control group and the intervention group, using the stratified randomization method considering the sex and GA levels.
Blood Sample Collection and Notification of the Results
All blood samples were collected in the Phase 1 Unit, the University of Tokyo Hospital. At screening, blood samples were collected from all participants to confirm whether the inclusion criteria had been met. After randomization, blood samples were collected from both participants of the control and intervention groups every 2 weeks over an 8-week period (visit 1–5). Participants of the control group were notified of the results of all the measurements conducted in the study only at the end of the study (visit 5); participants of the intervention group were notified of their GA levels each time on the same day as their blood samples were collected (visit 1–5) (Supplementary Table 1). During the 8-week intervention period, we only notified the intervention group of the results using paper on which each GA level was printed, and the participants continued to receive treatment from their primary care physician before the start of the study.
Body Weight Measurement, Questionnaire Evaluations, and Urine Test
All participants underwent body weight measurement at every visit (1–5). Each participant of both the control and intervention groups was notified of their body weight immediately after it was measured. The participants’ physical activity levels were analyzed using the Long-Form International Physical Activity Questionnaire (IPAQ), and the participants’ eating behaviors were analyzed using the YN Eating Behavior Questionnaire at visits 1 and 5. Urine samples were collected from both groups at visits 1 and 5, and the study participants were notified of the results of the urine tests only at the end of the study (Supplementary Table 1).
Body Composition Measurement
The body composition of the participants was measured at visits 1 and 5 (Supplementary Table 1) using a bioelectrical impedance analysis system (InBody 720, InBodyJapan). This system measures the impedance, and skeletal muscle mass and fat mass are calculated.
Change in Medication
Participants were withdrawn from the study if their medication was changed during the study period, because GA levels are affected by changes in the medication.
Study Endpoints
The primary endpoints were change in HbA1c, GA, and fasting plasma glucose level from baseline (visit 1) to visit 5. Secondary endpoints included changes from baseline (visit 1) to visit 5 in the following: body weight, waist circumference, body composition, lipid profile (plasma total cholesterol, triglyceride, HDL cholesterol, and non-HDL cholesterol levels), hepatic parameters (plasma AST, ALT, and γ-GTP levels), physical activity level, and eating behavior.
Statistical Analyses
Age, body mass index (BMI), and laboratory data other than overt proteinuria at visit 1 were expressed as the means ± SE and compared between the control and intervention groups by Student’s t test. The change of each parameter was calculated relative to the value at visit 1, and the change was compared between the control and intervention group by Student’s t test. The trend of each GA level from visit 1 to visit 5 was analyzed using the Jonckheere-Terpstra trend test. In the IPAQ analysis, the product of METs and exercise time (hours) per week was calculated and used as the physical activity level. In the YN Eating Behavior Questionnaire, statistical analyses were performed after the scores were aggregated by gender and converted into percentages. All the statistical analyses were carried out using the Statistical Package for Social Sciences (SPSS) version 22.0 (IBM Corp., Chicago, USA). A significance level of α = 0.05 was set for all the analyses.
Results
Enrollment and Outcomes
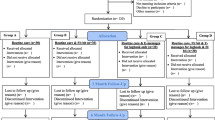
In September 2019, 63 participants with type 2 diabetes were enrolled in the study. The main exclusion criteria were unacceptably low or high HbA1c levels (two participants were excluded on the basis of this criterion). We assigned 31 participants to the control group and 30 participants to the intervention group. The actual intervention period was from October 1, 2019 to November 28, 2019. Two participants of the control group were excluded from the study as they were hospitalized for other diseases, while none of the participants of the intervention group was excluded from the study (Fig. 1).
Characteristics of the Participants
We assigned 21 men and 10 women to the control group and 21 men and 9 women to the intervention group. There were no statistically significant differences in age, BMI, fasting plasma glucose level, HbA1c, GA, lipid profile, albumin, HOMA-IR, HOMA-β, or renal function parameters between the control and intervention groups. Hepatic function parameters, especially the ALT levels, tended to be higher in the intervention group as compared with the control group (Table 1).
Changes in Body Weight, Waist Circumference, and Body Composition
The mean body weight changed from 67.4 kg at the baseline (visit 1) to 68.0 kg at visit 5 in the control group, and from 69.9 kg at the baseline to 69.9 kg at visit 5 in the intervention group; thus, the changes in the body weight (ΔBody weight) were 0.5 kg and − 0.0 kg, respectively, being 0.5 kg lower in the intervention group (P < 0.05) (Fig. 2A). At visit 2 also, the mean ΔBody weight was also 0.4 kg lower in the intervention group as compared with the control group (P < 0.05) (Fig. 2A).
Changes due to the intervention. A Body weight (kg), B waist circumference (cm), C body composition (%), D GA level (%), E fasting plasma glucose level (mg/dL), F HbA1c level (%), G total cholesterol level (mg/dL), H triglyceride level (mg/dL), I HDL cholesterol level (mg/dL), J non-HDL cholesterol level (mg/dL), K AST level (IU/L), L ALT level (IU/L), M γ-GTP level (IU/L), N physical activity level (MET hours/week), O eating behavior. *P < 0.05
The mean waist circumference changed from 90.2 cm at the baseline (visit 1) to 90.2 cm at visit 5 in the control group, and from 92.5 cm at the baseline to 92.3 cm at visit 5 in the intervention group; thus, the changes in the waist circumference (ΔWaist circumference) were 0.0 cm and − 0.3 cm, respectively, with no statistically significant difference in the (ΔWaist circumference) at visit 5 between the control and intervention groups (Fig. 2B).
The mean percent body fat changed from 30.3% at the baseline (visit 1) to 29.9% at visit 5 in the control group, and from 31.2% at the baseline to 30.0% at visit 5 in the intervention group; thus, the changes in the percent body fat (Δ%Body fat) were − 0.4% and − 1.2%, respectively. The mean percent body muscle changed from 38.1% at the baseline (visit 1) to 38.5% at visit 5 in the control group, and from 37.7% at the baseline to 38.6% at visit 5 in the intervention group; thus, the differences in the percent body muscle (Δ%Body muscle) were 0.4% and 0.9%, respectively. Thus, the mean Δ%Body fat was 0.8% lower and the mean Δ%Body muscle was 0.5% higher in the intervention group as compared with the control group (both, P < 0.05) (Fig. 2C).
Change in the Glycemic Biomarkers
The mean GA level changed from 18.2% at the baseline (visit 1) to 18.9% at visit 5 in the control group, and from 18.5% at the baseline to 18.9% at visit 5 in the intervention group; thus, the changes in the GA levels (ΔGA) were 0.7% and 0.3%, respectively, being 0.4% lower in the intervention group as compared with the control group (P = 0.40) (Fig. 2D). The Jonckheere-Terpstra trend test for ΔGA showed an increasing trend in the control group (p trend < 0.001), but not in the intervention group (p trend = 0.31).
The mean fasting glucose level changed from 140 mg/dL at the baseline (visit 1) to 140 mg/dL at visit 5 in the control group, and from 140 mg/dL at the baseline to 150 mg/dL a visit 5 in the intervention group; thus, the changes in the FPG (ΔFPG) were + 1 mg/dL and 9 mg/dL, respectively, with no statistically significant difference in the ΔFPG at visit 5 between the control and intervention groups (P = 0.440) (Fig. 2E).
The mean HbA1c level changed from 7.0% at the baseline (visit 1) to 7.1% at visit 5 in the control group, and from 7.1% at the baseline to 7.2% at visit 5 in the intervention group; thus, the changes in the HbA1c (ΔHbA1c) were 0.2% and 0.1%, respectively, with no statistically significantly difference in the ΔHbA1c at visit 5 between the control and intervention groups (Fig. 2F).
Changes in the Lipid Profile
The changes from the baseline (visit 1) to visit 5 in the serum total cholesterol (ΔTC), triglyceride (ΔTG), HDL cholesterol (ΔHDL-C), and non-HDL cholesterol (ΔnHDL-C) levels were not significantly different between the control and intervention groups (Fig. 2G–J).
Changes in Hepatic Parameters
The mean serum AST, ALT, and γ-GTP levels at visit 5 tended to decrease more in the intervention group as compared with the control group (P = 0.115, P = 0.297, and P = 0.068, respectively) (Fig. 2K–M).
Changes in the Physical Activity Level
The changes from the baseline (visit 1) to visit 5 in the physical activity level (ΔPhysical activity) tended to be higher in the intervention group as compared with the control group (P = 0.174) (Fig. 2N).
Changes in Eating Behavior
In regard to the eating behavior, there was no significant difference in the change from the baseline (visit 1) to visit 5 in the constitution (ΔConstitution) or content (ΔContents) between the intervention group and control group (P = 0.050 and P < 0.05, respectively) (Fig. 2O). The change from the baseline (visit 1) to visit 5 in the total score on the YN Eating Behavior Questionnaire (ΔTotal score) was also not significantly different between the intervention and control groups (P < 0.05) (Fig. 2O).
Discussion
Bi-weekly GA Measurement had a Positive Effect on the Eating Behavior and Physical Activity Level of the Participants, Which may Lead to Improvements in Diabetes Management and Obesity-Related Diseases
This study was conducted to evaluate how bi-weekly GA measurement might affect the participants’ eating behavior and physical activity level as assessed using self-administered questionnaires. Evaluation using the YN Eating Behavior Questionnaire showed significant improvements in the score on the dietary content and total score in the intervention group (Fig. 2O). Although no significant difference in the change of the physical activity level as assessed by the physical activity questionnaire was noted between the intervention and control groups, almost no decrease in the physical activity level was observed in the intervention group, while a decrease in the physical activity level was observed in the control group (Fig. 2N). These behavioral changes may have had a positive impact on body weight (visit 3 and visit 5), body composition, and hepatic parameters (Fig. 2A, C, D, M). However, it must be considered that because of the random assignment, the intervention group showed a bias in the mean γ-GTP versus the control group (P = 0.09). Our results suggest that bi-weekly GA measurement could improve the liver function, but a more homogeneous population study on the liver function would be needed to draw definitive conclusions. The t tests for changes in the GA, FPG, and HbA1c showed no significant differences between the control and intervention groups (Fig. 2D–F). However, the Jonckheere-Terpstra trend test for ΔGA (%) showed an increasing trend in the control group (p trend < 0.001), but not in the intervention group (p trend = 0.31). These results suggest that bi-weekly GA measurement may have a positive impact on glycemic control.
Strength of this Study and Comparison with Previous Studies
The strength of this study lies in the fact that regular GA measurements induced behavioral changes and improved body weight, diabetes, and obesity-related diseases for the first time. SMBG and CGM have reported that visualizing blood glucose levels improves glycemic control in people with diabetes [7,8,9]. In this study, blood sampling was performed only once every 2 weeks, whereas frequent finger pricks or placement of a sensor under the skin was needed in previous studies. Additionally, the intervention period in this study was 8 weeks, shorter than the intervention periods ranging from 6 months to 1 year in some previous studies. These findings suggest that our intervention method may yield results at an earlier stage with minimally invasive approaches.
Limitations of this Study
This was a single-center study, not blinded, and open label. The medication history, comorbidities, disease duration, family history, incomes, smoking status, education levels, and lifestyles of the participants were unknown.
Future Prospects
In this study, GA levels were measured bi-weekly, and the results were provided on printed sheets. The frequency of measurements and the method of notification are topics of debate. In particular, regarding the method of notification, there is a possibility that greater behavioral change can be induced by creating graphs of measurement results and setting target GA levels including smartphone utilization. Recently, it has also been reported that the GA levels can be measured non-invasively using tears and saliva [25], and it is expected that the burden on people with diabetes could be reduced. Additionally, there is also a recent report on the establishment of a method to measure GA levels in self-sampled, finger-prick blood sent through postal services [26], and the intervention implemented in this study could be feasible without requiring hospital visits.
Conclusion
Bi-weekly measurement and prompt notification of the GA over an 8-week period in people with type 2 diabetes significantly improved their eating behavior, exercise habits, and weight and body composition, and might have a positive impact on the glycemic control.
References
Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–9.
Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. https://doi.org/10.1136/bmj.321.7258.405.
Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197–206. https://doi.org/10.1056/NEJMoa1414266.
Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85. https://doi.org/10.2337/dc10-0666.
Sasazuki S, Charvat H, Hara A, et al. Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci. 2013;104(11):1499–507. https://doi.org/10.1111/cas.12241.
Cukierman-Yaffe T, Gerstein HC, Williamson JD, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009;32(2):221–6. https://doi.org/10.2337/dc08-1153.
Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262–7. https://doi.org/10.2337/dc10-1732.
Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;5(1):39. https://doi.org/10.1186/1758-5996-5-39.
Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262–72. https://doi.org/10.1001/jama.2021.7444.
Malanda UL, Welschen LM, Riphagen II, et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;1:CD005060. https://doi.org/10.1002/14651858.CD005060.pub3.
Ramchandani N, Arya S, Ten S, Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J Diabetes Sci Technol. 2011;5(4):860–70. https://doi.org/10.1177/193229681100500407.
Divan V, Greenfield M, Morley CP, Weinstock RS. Perceived burdens and benefits associated with continuous glucose monitor use in type 1 diabetes across the lifespan. J Diabetes Sci Technol. 2022;16(1):88–96. https://doi.org/10.1177/1932296820978769.
Polonsky WH, Fisher L. Self-monitoring of blood glucose in noninsulin-using type 2 diabetic patients: right answer, but wrong question: self-monitoring of blood glucose can be clinically valuable for noninsulin users. Diabetes Care. 2013;36(1):179–82. https://doi.org/10.2337/dc12-0731.
Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27–S34. https://doi.org/10.1089/dia.2021.0007.
Furusyo N, Hayashi J. Glycated albumin and diabetes mellitus. Biochim Biophys Acta. 2013;1830(12):5509–14. https://doi.org/10.1016/j.bbagen.2013.05.010.
Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18(4):440–7. https://doi.org/10.2337/diacare.18.4.440.
Koga M. Glycated albumin; clinical usefulness. Clin Chim Acta. 2014;433:96–104. https://doi.org/10.1016/j.cca.2014.03.001.
Zendjabil M. Glycated albumin. Clin Chim Acta. 2020;502:240–4. https://doi.org/10.1016/j.cca.2019.11.007.
Ashby W. The span of life of the red blood cell; a resume. Blood. 1948;3(5):486–500.
Zhang HD, Ma YJ, Liu QF, et al. Human erythrocyte lifespan measured by Levitt’s CO breath test with newly developed automatic instrument. J Breath Res. 2018;12(3): 036003. https://doi.org/10.1088/1752-7163/aaacf1.
Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63(12):1469–79. https://doi.org/10.1016/j.metabol.2014.08.010.
Ahmadieh H, Azar ST. Liver disease and diabetes: association, pathophysiology, and management. Diabetes Res Clin Pract. 2014;104(1):53–62. https://doi.org/10.1016/j.diabres.2014.01.003.
Sakura H, Tanaka Y, Iwamoto Y. Seasonal fluctuations of glycated hemoglobin levels in Japanese diabetic patients. Diabetes Res Clin Pract. 2010;88(1):65–70. https://doi.org/10.1016/j.diabres.2009.12.011.
Sakamoto M, Matsutani D, Minato S, et al. Seasonal variations in the achievement of guideline targets for HbA(1c), blood pressure, and cholesterol among patients with type 2 diabetes: a nationwide population-based study (ABC Study: JDDM49). Diabetes Care. 2019;42(5):816–23. https://doi.org/10.2337/dc18-1953.
Aihara M, Jinnouchi H, Yoshida A, et al. Evaluation of glycated albumin levels in tears and saliva as a marker in patients with diabetes mellitus. Diabetes Res Clin Pract. 2023;199: 110637. https://doi.org/10.1016/j.diabres.2023.110637.
Aihara M, Irie T, Yasukawa K, et al. Development of a high-performance liquid chromatographic glycated albumin assay using finger-prick blood samples. Clin Chim Acta. 2023;542: 117272. https://doi.org/10.1016/j.cca.2023.117272.
Acknowledgements
We thank all the participants for their cooperation in the study.
Funding
This study and the journal’s Rapid Service fee were supported by a grant from the Japan Agency for Medical Research and Development (AMED) (Grant No. 20hm0102065h0003).
Medical Writing, Editorial, and Other Assistance
We thank all the staff members of the Phase 1 unit, the University of Tokyo Hospital, for their excellent assistance.
Author Contribution
Conceptualization: Masakazu Aihara, Naoto Kubota; Methodology: Masakazu Aihara, Naoto Kubota; Formal analysis and investigation: Masakazu Aihara, Naoto Kubota; Writing: Masakazu Aihara, Naoto Kubota; Discussion: Masakazu Aihara, Takanori Hayashi, Chie Koizumi, Yoshitaka Sakurai, Tetsuya Kubota, Takashi Kadowaki, Tosimasa Yamauchi, Naoto Kubota; Data collection: Masakazu Aihara, Mika Sawada.
Disclosures
Masakazu Aihara, Takanori Hayashi, Chie Koizumi, Yoshitaka Sakurai, Mika Sawada, Tetsuya Kubota, Takashi Kadowaki, Toshimasa Yamauchi, and Naoto Kubota have no competing interests to report that are relevant to the publication of this article.
Compliance with Ethics Guidelines
The study protocol was approved by the Research Ethics Committee of the University of Tokyo Hospital (2019092NI), and the study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent to this study was obtained from all the study participants after they were provided an explanation about the study.
Data Availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Aihara, M., Hayashi, T., Koizumi, C. et al. Bi-weekly Glycated Albumin Measurement was Useful to Encourage Behavioral Changes in People with Type 2 Diabetes Mellitus. Diabetes Ther 14, 1711–1721 (2023). https://doi.org/10.1007/s13300-023-01452-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01452-y