Abstract
Introduction
This study explored the correlation between sex hormones, sex hormone binding globulin (SHBG), and insulin resistance in male patients with newly diagnosed type 2 diabetes mellitus (T2DM).
Methods
A total of 48 male patients with newly diagnosed T2DM were enrolled in this study between March 2022 and December 2022. Clinical characteristics, sex hormones, and SHBG levels were collected. All enrolled subjects received intensive hypoglycemic treatment with insulin pump for 1 week to achieve glycemic control, then the steady-state glucose infusion rate (GIR), an indicator of insulin sensitivity, was determined by the hyperinsulinemic-euglycemic clamp. Correlation analysis and multivariate logistic regression analysis were performed to explore the association of clinical characteristics, sex hormones, and SHBG with insulin sensitivity. The optimal cutoff value to predict insulin resistance was calculated using receiver operating characteristic (ROC) curve.
Results
According to the GIR cut-point value of 5.700 mg/(kg min), there were 40 patients with insulin resistance (IR group) and 8 patients without (non-IR group). The IR group exhibited lower testosterone and SHBG levels than the non-IR group (all p < 0.050). Correlation analysis showed that insulin sensitivity was positively associated with testosterone and SHBG, while negatively associated with body mass index, fasting blood glucose, alanine aminotransferase, aspartate aminotransferase, total cholesterol, triglyceride, and apolipoprotein B (all p < 0.050). Multivariate logistic regression analysis demonstrated that SHBG is an independent predictor for insulin resistance (p = 0.029). Further ROC curve analysis revealed that the optimal cutoff value of SHBG to predict insulin resistance is 17.200 nmol/L, with the corresponding area under the curve (AUC) and its 95% confidence interval (CI) being 0.813 and 0.691–0.934.
Conclusions
SHBG is an independent predictor for insulin resistance in male patients with newly diagnosed T2DM.
Trial Registration Number
KY20220314-01.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Low levels of testosterone and sex hormone binding globulin (SHBG) are significantly associated with increased risk of type 2 diabetes mellitus (T2DM). |
The relationships between sex hormones, SHBG, and insulin resistance remain inconsistent, and very limited data are available in male patients with newly diagnosed T2DM. |
This study was aimed to investigate the association of circulating sex hormones, SHBG, and insulin resistance evaluated by glucose infusion rate (GIR) using the hyperinsulinemic-euglycemic clamp in male patients with newly diagnosed T2DM. |
What was learned from the study? |
Male patients with newly diagnosed T2DM and insulin resistance showed lower testosterone and SHBG levels. |
SHBG was an independent predictor for insulin resistance in male patients with newly diagnosed T2DM, with the optimal cutoff value being 17.200 nmol/L. |
Introduction
Diabetes mellitus is a chronic metabolic disorder worldwide. There were about 536.6 million people suffering from diabetes in 2021, and this number is expected to be 783.7 million by 2045 according to the latest International Diabetes Federation (IDF) data [1]. Among them, more than 90% have type 2 diabetes mellitus (T2DM), which is characterized by insulin resistance and relative insulin deficiency.
Testosterone is the primary male sex hormone which plays an important role in regulating sexual maturation and fertility, as well as being involved in carbohydrate, fat, and protein metabolism [2]. Sex hormone binding globulin (SHBG) is a glycoprotein produced by the liver and traditionally known as a carrier protein for sex hormones in the circulation [3]. Previous cross-sectional studies have reported a higher prevalence of low testosterone and SHBG levels in men with T2DM [4,5,6]. Longitudinal studies demonstrated that men with low levels of testosterone and SHBG are significantly associated with increased risk of T2DM [7,8,9,10,11]. Similar results also revealed that low testosterone or SHBG levels can be a risk marker for the development of T2DM in women [7, 9, 12, 13].
Although the exact mechanisms behind the association between testosterone, SHGB, and type 2 diabetes are not fully understood, insulin resistance may mediate them in part. However, the relationship between sex hormones, SHBG, and insulin resistance remain inconsistent, and very limited data are available in male patients with newly diagnosed T2DM. Studies on the cutoff value for predicting insulin resistance in male patients with newly diagnosed T2DM are lacking. Therefore, we performed this study to investigate the association of circulating sex hormones, SHBG, and insulin resistance evaluated by glucose infusion rate (GIR) using the hyperinsulinemic-euglycemic clamp in male patients with newly diagnosed T2DM.
Methods
Study Design
This study protocol was approved by the Institutional Ethical Committee of Nanjing First Hospital in accordance with the Declaration of Helsinki guidelines (KY20220314-01). All patients provided their written informed consent to participate. Briefly, male, drug-naive patients with newly diagnosed T2DM and aged 18–60 years were recruited into this study. The diagnostic criteria of diabetes were based on a plasma fasting glucose level ≥ 7.0 mmol/L, 2 h oral glucose tolerance test (OGTT) values ≥ 11.1 mmol/L, or random glucose level ≥ 11.1 mmol/L recommended by World Health Organization 1999 criteria. The exclusion criteria were (a) patients with acute complication of diabetes, (b) patients with an acute infection or stress state, (c) patients with secondary hypogonadism, (d) patients used systemic steroidal anti-inflammatory drugs or any drugs that may influence testosterone level in the last 3 months, and (e) patients with a severe systemic disease or any other condition that is judged by researchers to be unsuitable for this study. Finally, a total of 48 male patients with newly diagnosed T2DM admitted to Nanjing First Hospital between March 2022 and December 2022 were enrolled in this study.
Clinical Data Collection
Anthropometric parameters such as age, weight, height, and blood pressure were recorded upon entry into the study. Body mass index (BMI) was calculated as weight divided by the square of height (kg/m2). Blood samples of all enrolled subjects were collected after overnight fasting (> 10 h). Fasting blood glucose (FBG), liver and renal functions involving alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (Alb), and creatinine (Cr), and lipid profiles such as total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), apolipoprotein A1 (ApoA1), and apolipoprotein B (ApoB) were analyzed by standard enzymatic assays (Olympus AU5400 autoanalyzer; Beckman Coulter, Japan). Glycated hemoglobin (HbA1c) and fasting C‐peptide (FCP) were measured using high‐performance liquid chromatography assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and chemiluminescent immunometric assay (Modular Analytics E170; Roche Diagnostics GmbH, Mannheim, Germany), respectively. Circulating sex hormones, including estradiol (E2), testosterone (T), prolactin (PRL), dehydroepiandrosterone (DHEA), luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and SHBG were measured using chemiluminescent immunometric assay (UniCel™ DxI 800 automated analyzer; Beckman Coulter Inc., Brea, CA, USA).
Insulin Sensitivity Assessment
All hospitalized male patients with newly diagnosed T2DM who met the inclusion and exclusion criteria received intensive hypoglycemic treatment with insulin pump for 1 week to achieve glycemic control. The standard of glycemic control was defined as more than 80% of FBG < 7.0 mmol/L and 2 h postprandial blood glucose < 11.1 mmol/L using the flash glucose monitoring (FGM) system. The insulin pump was stopped 12 h before the clamp start, and insulin sensitivity was then evaluated with GIR using the hyperinsulinemic-euglycemic clamp, which is the gold standard for assessing insulin sensitivity [14]. After an approximately 12-h overnight fast, subjects arrived at the laboratory at 8 a.m. An intravenous cannula was inserted into the right antecubital fossa vein for blood sampling, and another into the left antecubital fossa vein for insulin and glucose infusion. Regular human insulin was infused according to the target plasma glucose level and maintained at a continuous rate of 1.500 mU/kg/min along with an intravenous 20% glucose infusion for 120 min. The plasma glucose level was detected every 5 min, and kept at the target value of 90 mg/dl (5.0 mmol/L) finally. Insulin-stimulated glucose disposal rates (M value) were calculated as the GIR per kg per minute (mg/kg/min) during the last 20 min of steady state [15].
Statistical Analysis
All quantitative data were checked for normality by one-sample Kolmogorov–Smirnov (K-S) test, and then presented as mean ± standard deviation or median (interquartile range) as appropriate. The normally distributed data were compared using Student’s t test, whereas the asymmetrically distributed data were compared using Mann–Whitney U test. The categorical variables were shown as n (%), and compared with Pearson’s chi-squared (χ2) test. Correlation analysis was used to investigate the relationship of clinical characteristics, sex hormones, and SHBG with insulin resistance. Multivariate logistic regression analysis was performed to determine independent factors associated with the susceptibility to insulin resistance. The optimal logistic regression model with a stepwise forward method through a likelihood-ratio test was established by including all risk factors, with insulin resistance as the prediction. Receiver operating characteristic (ROC) curve analysis was employed to determine the area under the curve (AUC) with corresponding 95% confidence interval (CI), and the Youden index was calculated to identify the optimal cutoff point for predicting insulin resistance, as well as its sensitivity and specificity. All statistical analyses were performed with SPSS version 22.0 (SPSS Inc., Chicago, IL, USA), and a two-side p value < 0.050 was considered statistically significant.
Results
Clinical Characteristics of the Enrolled Subjects
A total of 48 male patients with newly diagnosed T2DM were enrolled in this study, and their clinical characteristics are summarized in Table 1. According to the GIR cut-point value of 5.700 mg/(kg min), there were 40 patients with the insulin resistant (IR group) and 8 patients with insulin sensitivity (non-IR group) [16]. The two groups were well matched in age, BMI, and history of hypertension (all p > 0.050). AST and TG levels were higher in the IR group when compared to the non-IR group (p = 0.034 and 0.017, respectively). There were no between-group differences in FBG, HbA1c, FCP, ALT, Alb, Cr, TC, TG, HDL, LDL, ApoA1, ApoB, and ApoB/ApoA1 (all p > 0.050).
Circulating Sex Hormones and SHBG Levels Between Groups
Levels of circulating sex hormones and SHBG are summarized in Table 2. The IR group had lower testosterone and SHBG levels than the non-IR group (p = 0.046 and 0.006, respectively). There were no differences in E2, PRL, DHEA, LH, and FSH between the two groups (all p > 0.050).
Correlation Between Clinical Characteristics, Sex Hormones, SHBG, and Insulin Resistance in Male Patients with Newly Diagnosed T2DM
In order to explore the correlation between clinical characteristics, sex hormones, SHBG, and insulin resistance, Spearman correlation analysis was performed. GIR was positively associated with testosterone (r = 0.290, p = 0.046) and SHBG (r = 0.444, p = 0.002), but negatively associated with BMI (r = − 0.394, p = 0.006), FBG (r = − 0.329, p = 0.024), ALT (r = − 0.325, p = 0.024), AST (r = − 0.372, p = 0.009), TC (r = − 0.370, p = 0.010), TG (r = − 0.541, p < 0.001), and ApoB (r = − 0.327, p = 0.025) (Table 3). As GIR is negatively related to insulin resistance, the results suggested that insulin resistance is negatively correlated to testosterone and SHBG, while positively correlated to BMI, FBG, ALT, AST, TC, TG, and ApoB.
Logistic Regression Analysis to Identify Independent Contributors to Insulin Resistance in Male Patients with Newly Diagnosed T2DM
As correlation analysis suggested that testosterone, SHBG, BMI, FBG, ALT, AST, TC, TG, and ApoB are associated with insulin resistance, these factors were further included in the multivariate logistic regression analysis with a stepwise forward method through a likelihood-ratio test to identify independent contributors to insulin resistance. Results demonstrated that only SHBG independently contributed to insulin resistance in male patients with newly diagnosed T2DM, with corresponding OR (95% CI) being 1.104 (1.010–1.208) and p value being 0.029 (Table 4).
ROC Analysis to Identify the Optimal Cutoff Value of SHBG Predicting Insulin Resistance in Male Patients with Newly Diagnosed T2DM
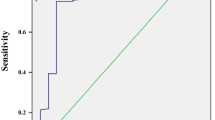
According to the results of multivariate logistic regression analysis, SHBG was a predictive factor of insulin resistance in male patients with newly diagnosed T2DM. ROC analysis and the Youden index were employed to identify the optimal cutoff value of SHBG to predict insulin resistance. The corresponding AUC and its 95% CI were 0.813 and 0.691–0.934, with the optimal SHBG cutoff value predicting insulin resistance being 17.200 nmol/L (Fig. 1).
Receiver operating characteristic (ROC) curve employed to identify the optimal cutoff value of SHBG to predict insulin resistance in male patients with newly diagnosed T2DM. Area under the curve (95% confidence interval) 0.813 (0.691–0.934), cutoff value 17.200 nmol/L, Youden index 0.675, sensitivity 100.000%, specificity 67.500%
Discussion
In the present study, male patients with newly diagnosed T2DM and insulin resistance exhibited higher AST and TG levels, but lower testosterone and SHBG levels than those without insulin resistance. Correlation analysis showed that insulin sensitivity was positively associated with testosterone and SHBG. Further multivariate logistic regression analysis and ROC analysis revealed that circulating SHBG level was independently negatively associated with insulin resistance, and could be a predictor of insulin resistance with the optimal cutoff value of SHBG being 17.200 nmol/L in male patients with newly diagnosed T2DM.
Consistent with a previous study, there was a stronger correlation between SHBG and insulin resistance than for testosterone and insulin resistance [17]. Joyce et al. also demonstrated that SHBG and dihydrotestosterone, but not free testosterone (FT), are inversely associated with insulin resistance [18]. In a study published in Diabetes Care, the association between SHBG and insulin resistance was shown to be independent of total testosterone (TT), while the association between TT and insulin resistance was mediated by SHBG [19]. SHBG was also proved to be a significant determinant of homeostasis model assessment of insulin resistance (HOMA-IR) independent of body fat, while the inverse association between testosterone and insulin resistance was mediated through body fat [20]. In the Postmenopausal Estrogen/Progestin Intervention Trial, lower SHBG rather than testosterone level was linked to higher odds of insulin resistance among postmenopausal women [21]. Regardless of menopausal state, SHBG level was also demonstrated to predict the development of insulin resistance in both men and women [22]. A cross-sectional study involving 334 men with nonalcoholic fatty liver disease (NAFLD) revealed that SHBG is independently associated with HOMA-IR even adjusting for potential confounders [23]. However, there also existed inconsistent results that both lower concentrations of TT and SHBG are inversely correlated with HOMA-IR [24, 25]. Osuna et al. also reported that TT and SHBG concentrations proportionally diminished with the increase of insulin resistance index in men with obesity [26]. Low testosterone levels were proved to be independently associated with insulin resistance in men both with and without diabetes [4, 27, 28]. In elderly male patients with T2DM and osteoporosis, the reduced level of serum testosterone might promote insulin resistance [29]. Empirical optimal cutoff value for SHBG level was demonstrated to be ≤ 41.5 nmol/L typical for insulin resistance in 854 patients with polycystic ovary syndrome (PCOS) [30]. Among patients with insulin resistance defined as HOMA-IR ≥ 2.29, the optimal SHBG cutoff values for predicting insulin resistance in the non-overweight group, overweight/obese group, and all patients with PCOS were 38.4, 37.0, and 38.4 nmol/L, respectively; the corresponding values were 37.6, 30.2, and 30.2 nmol/L among patients with insulin resistance defined as HOMA-IR ≥ 2.5, respectively [31]. However, the reasons for the inconsistent results are not entirely clear. Possibly, different disease populations, ethnicity, and sample size of enrolled subjects may contribute to the inconsistency in these findings. Additionally, the methods for assessing insulin sensitivity varied from study to study, which may be partly responsible.
However, the exact mechanisms linking SHBG to insulin resistance were still not well understood. In vitro study showed that SHBG could suppress inflammation and lipid deposition, which might mediate the protective effect of SHBG against the incidence of metabolic syndrome [32]. In a cellular model of metabolic dysregulation, SHBG was found to mitigate palmitate-induced lipotoxicity and endoplasmic reticulum (ER) stress, which was also demonstrated in the liver of patients with metabolic syndrome [33]. Both aforementioned inflammation and ER stress have been associated with hepatic insulin resistance [34]. Moreover, SHBG may regulate the activity of the extracellular signal-regulated kinase (ERK) pathway in order to participate in the onset and development of insulin resistance [35]. In a cellular model, SHBG could induce insulin resistance and gestational diabetes mellitus (GDM) via regulating glucose transporter 1 (GLUT1) expression through the cAMP/PKA/CREB1 pathway [36]. Low SHBG expression was also demonstrated to exert great effects on local insulin resistance, as well as the PI3K/AKT pathway-mediated systemic insulin resistance in cellular models [37].
To the best of our knowledge, this study is the first to investigate the association between circulating sex hormones, SHBG, and insulin resistance in male patients with newly diagnosed T2DM after achieving glycemic control. Moreover, hyperinsulinemic-euglycemic clamp, generally accepted as the gold standard method, has been utilized to assess insulin sensitivity in this study [38]. Moreover, insulin sensitivity was evaluated after 1-week intensive hypoglycemic treatment with insulin pump to achieve glycemic control, which could avoid the influence of hyperglycemia on insulin sensitivity. However, certain limitations should be discussed. First, this is a cross-sectional study, and we can not draw a causal role for SHBG in the pathogenesis of insulin resistance. Second, FT or bioavailable testosterone which truly reflects the testosterone level that exerts biological effects was not examined in this study. Additionally, our study was performed in a small number of enrolled subjects and a singer center because of the complexity and high cost of the hyperinsulinemic-euglycemic clamp, which limited the power of our results to a certain degree.
Conclusion
SHBG is an independent predictor for insulin resistance in male patients with newly diagnosed T2DM. Further well-designed prospective cohort studies are warranted to confirm this observed association, and to determine the causality of SHBG with the onset and progression of insulin resistance in male patients with newly diagnosed type 2 diabetes mellitus.
References
Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. https://doi.org/10.1016/j.diabres.2021.109119.
Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25-45. https://doi.org/10.1530/JOE-12-0455.
Shah W, Khan R, Shah B, et al. The molecular mechanism of sex hormones on Sertoli cell development and proliferation. Front Endocrinol (Lausanne). 2021;12:648141. https://doi.org/10.3389/fendo.2021.648141.
Sepu N, Adeleye JO, Kuti MO. Serum testosterone in Nigerian men with type 2 diabetes mellitus and its relationship with insulin sensitivity and glycemic control. J Natl Med Assoc. 2021;113(3):285–93. https://doi.org/10.1016/j.jnma.2020.11.014.
Paruk IM, Pirie FJ, Nkwanyana NM, Motala AA. Prevalence of low serum testosterone levels among men with type 2 diabetes mellitus attending two outpatient diabetes clinics in KwaZulu-Natal Province. South Africa S Afr Med J. 2019;109(12):963–70. https://doi.org/10.7196/SAMJ.2019.v109i12.013893.
Zhang J, Li X, Cai Z, Li H, Yang B. Association between testosterone with type 2 diabetes in adult males, a meta-analysis and trial sequential analysis. Aging Male. 2020;23(5):607–18. https://doi.org/10.1080/13685538.2018.1557139.
O’Reilly MW, Glisic M, Kumarendran B, et al. Serum testosterone, sex hormone-binding globulin and sex-specific risk of incident type 2 diabetes in a retrospective primary care cohort. Clin Endocrinol (Oxf). 2019;90(1):145–54. https://doi.org/10.1111/cen.13862.
Lakshman KM, Bhasin S, Araujo AB. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. J Gerontol A Biol Sci Med Sci. 2010;65(5):503–9. https://doi.org/10.1093/gerona/glq002.
Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361(12):1152–63. https://doi.org/10.1056/NEJMoa0804381.
Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27(5):1036–41. https://doi.org/10.2337/diacare.27.5.1036.
Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23(4):490–4. https://doi.org/10.2337/diacare.23.4.490.
Saikia UK, Jabbar PK, Das DV. Sex hormone-binding globulins and testosterone levels as a risk marker for type 2 diabetes mellitus among postmenopausal women. J Midlife Health. 2021;12(2):155–60. https://doi.org/10.4103/jmh.JMH_142_20.
Lindstedt G, Lundberg PA, Lapidus L, Lundgren H, Bengtsson C, Bjorntorp P. Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM. 12-yr follow-up of population study of women in Gothenburg, Sweden. Diabetes. 1991;40(1):123–8. https://doi.org/10.2337/diab.40.1.123.
Qian X, Wang H, Yang G, et al. Pioglitazone improved insulin sensitivity and first phase insulin secretion among obese and lean people with diabetes: a multicenter clamp study. Diabetes Ther. 2018;9(2):815–26. https://doi.org/10.1007/s13300-018-0401-9.
Jiang LL, Wang SQ, Ding B, et al. The effects of add-on exenatide to insulin on glycemic variability and hypoglycemia in patients with type 1 diabetes mellitus. J Endocrinol Invest. 2018;41(5):539–47. https://doi.org/10.1007/s40618-017-0765-0.
Cobb J, Gall W, Adam KP, et al. A novel fasting blood test for insulin resistance and prediabetes. J Diabetes Sci Technol. 2013;7(1):100–10. https://doi.org/10.1177/193229681300700112.
Endre T, Mattiasson I, Berglund G, Hulthen UL. Low testosterone and insulin resistance in hypertension-prone men. J Hum Hypertens. 1996;10(11):755–61.
Joyce KE, Biggs ML, Djousse L, et al. Testosterone, dihydrotestosterone, sex hormone-binding globulin, and incident diabetes among older men: the cardiovascular health study. J Clin Endocrinol Metab. 2017;102(1):33–9. https://doi.org/10.1210/jc.2016-2623.
Rajala UM, Keinanen-Kiukaanniemi SM, Hirsso PK, et al. Associations of total testosterone and sex hormone-binding globulin levels with insulin sensitivity in middle-aged Finnish men. Diabetes Care. 2007;30(4):e13. https://doi.org/10.2337/dc06-1979.
Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27(4):861–8. https://doi.org/10.2337/diacare.27.4.861.
Kalish GM, Barrett-Connor E, Laughlin GA, Gulanski BI, Postmenopausal Estrogen/Progestin Intervention Trial. Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab. 2003;88(4):1646–52. https://doi.org/10.1210/jc.2002-021375.
Ottarsdottir K, Hellgren M, Bock D, Nilsson AG, Daka B. Longitudinal associations between sex hormone-binding globulin and insulin resistance. Endocr Connect. 2020;9(5):418–25. https://doi.org/10.1530/EC-20-0141.
Ye J, Yao Z, Tan A, et al. Low serum sex hormone-binding globulin associated with insulin resistance in men with nonalcoholic fatty liver disease. Horm Metab Res. 2017;49(5):359–64. https://doi.org/10.1055/s-0043-102690.
Zhang J, Huang X, Liao M, et al. Both total testosterone and sex hormone-binding globulin are independent risk factors for metabolic syndrome: results from Fangchenggang Area Male Health and Examination Survey in China. Diabetes Metab Res Rev. 2013;29(5):391–7. https://doi.org/10.1002/dmrr.2405.
Li C, Ford ES, Li B, Giles WH, Liu S. Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care. 2010;33(7):1618–24. https://doi.org/10.2337/dc09-1788.
Osuna JA, Gomez-Perez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. 2006;52(5):355–61. https://doi.org/10.1080/01485010600692017.
Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93(5):1834–40. https://doi.org/10.1210/jc.2007-2177.
Yeap BB, Chubb SA, Hyde Z, et al. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the Health In Men Study. Eur J Endocrinol. 2009;161(4):591–8. https://doi.org/10.1530/EJE-09-0348.
Xia JW, Tan SJ, Zhang XL, Jun T, Sun XK, Ling W. Correlation of serum testosterone with insulin resistance in elderly male type 2 diabetes mellitus patients with osteoporosis. J Diabetes Investig. 2015;6(5):548–52. https://doi.org/10.1111/jdi.12291.
Biernacka-Bartnik A, Kocelak P, Owczarek AJ, et al. Prediction of insulin resistance and impaired fasting glucose based on sex hormone-binding globulin (SHBG) levels in polycystic ovary syndrome. Int J Endocrinol. 2022;2022:6498768. https://doi.org/10.1155/2022/6498768.
Chen F, Liao Y, Chen M, et al. Evaluation of the efficacy of sex hormone-binding globulin in insulin resistance assessment based on HOMA-IR in patients with PCOS. Reprod Sci. 2021;28(9):2504–13. https://doi.org/10.1007/s43032-021-00535-0.
Yamazaki H, Kushiyama A, Sakoda H, et al. Protective effect of sex hormone-binding globulin against metabolic syndrome: in vitro evidence showing anti-inflammatory and lipolytic effects on adipocytes and macrophages. Mediators Inflamm. 2018;2018:3062319. https://doi.org/10.1155/2018/3062319.
Kornicka-Garbowska K, Bourebaba L, Rocken M, Marycz K. Sex hormone binding globulin (SHBG) mitigates ER stress in hepatocytes in vitro and ex vivo. Cells. 2021. https://doi.org/10.3390/cells10040755.
Kim OK, Jun W, Lee J. Mechanism of ER stress and inflammation for hepatic insulin resistance in obesity. Ann Nutr Metab. 2015;67(4):218–27. https://doi.org/10.1159/000440905.
Wang X, Chi X, Feng C, Zhang X, Jin Z. Sex hormone-binding globulin regulates the activity of the ERK pathway in the placentas of patients with gestational diabetes mellitus. Biochem Biophys Res Commun. 2020;532(4):613–9. https://doi.org/10.1016/j.bbrc.2020.08.100.
Chi X, Feng C, Wang X, Jin Z. Sex hormone-binding globulin regulates glucose metabolism in human placental trophoblasts via cAMP/PKA/CREB1. J Obstet Gynaecol Res. 2020;46(11):2340–6. https://doi.org/10.1111/jog.14429.
Feng C, Jin Z, Chi X, et al. SHBG expression is correlated with PI3K/AKT pathway activity in a cellular model of human insulin resistance. Gynecol Endocrinol. 2018;34(7):567–73. https://doi.org/10.1080/09513590.2017.1411474.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. https://doi.org/10.1152/ajpendo.1979.237.3.E214.
Acknowledgements
We would like to express our heartfelt gratitude to the staff of the Department of Endocrinology, Nanjing First Hospital for their selfless help and valuable assistance. We also thank the participants of the study for their strong support.
Funding
This work and Rapid Service Fee were supported by the Project supported by Nanjing Medical Science and Technology Development Fund (No. ZKX22038) and the National Key R&D Program of China (No. 2018YFC1314100).
Medical Writing and Editorial Assistance
The authors did not receive any medical writing or editorial assistance for this article.
Author Contributions
Jianhua Ma and Bo Ding designed the study; Rong Huang, Ying Wang and Rengna Yan collected the data, performed the analyses and wrote the first draft; and Jianhua Ma and Bo Ding checked the manuscript and revised it. All authors approved the final submission.
Disclosures
The authors declare no conflicts of interest.
Compliance with Ethics Guidelines
This study protocol was approved by the Institutional Ethical Committee of Nanjing First Hospital in accordance with the Declaration of Helsinki guidelines (KY20220314-01). All patients provided their written informed consent to participate.
Data Availability
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Huang, R., Wang, Y., Yan, R. et al. Sex Hormone Binding Globulin is an Independent Predictor for Insulin Resistance in Male Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Diabetes Ther 14, 1627–1637 (2023). https://doi.org/10.1007/s13300-023-01445-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01445-x