Abstract
Background
Individuals initially diagnosed with type 2 diabetes (T2D) might exhibit positivity for diabetes-associated autoantibodies (DAA +). We investigated the prevalence of DAA positivity in a group of individuals with T2D who were referred to a tertiary diabetes centre within a pre-specified period of time. We aimed to identify characteristics linked with DAA positivity by comparing DAA + individuals with their DAA-negative counterparts.
Methods
This was a cross-sectional study into which all T2D patients referred to the National Institute of Endocrinology and Diabetology, Ľubochňa, Slovakia, between 1 January and 30 June 2016 were included. Data on > 70 participants’ characteristics, including antibodies against glutamic acid decarboxylase (anti-GAD65), insulinoma-associated antigen IA-2 (IA-2A) and insulin (IAA), were collected.
Results
Six hundred and ninety-two individuals (387, 55.6% female) with a median (range) age of 62 (24–83) years, HbA1c of 8.9 (5.0–15.7)% [74 (31–148 mmol/mol)] and diabetes duration of 13.0 (0–42) years were analysed. One hundred and forty-five (145/692, 21.0%) tested positive for at least one DAA; 136/692 (19.7%) were positive for anti-GAD65, 21/692 (3.0%) were positive for IA-2A and 9/692 (1.3%) were positive for IAA. Only 84.9% of the DAA + individuals aged > 30 years at the time of diabetes diagnosis met the current diagnostic criteria for latent autoimmune diabetes of adults (LADA). DAA + differed from DAA − individuals in multiple characteristics, including the incidence of hypoglycaemia.
Conclusion
Several pathological processes linked with distinct types of diabetes can develop in parallel, including insulin resistance and autoimmune insulitis. In this single-centre cross-sectional study from Slovakia, we report a higher than previously published prevalence of DAA positivity in a group of individuals with a formal diagnosis of T2D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Individuals initially diagnosed with type 2 diabetes (T2D) might exhibit positivity for diabetes-associated autoantibodies (DAA +). |
We investigated the prevalence of DAA positivity in a group of individuals with T2D referred to a tertiary diabetes centre in Slovakia within a pre-specified period of time. |
Approximately 1 in 5 individuals with a formal diagnosis of T2D were found to be positive for at least one of the tested DAA (anti-GAD65, IA-2A or IAA). |
Only approximately 85% of the DAA + individuals aged > 30 years at the time of diabetes diagnosis met the current diagnostic criteria for latent autoimmune diabetes of adults (LADA). |
Several pathological processes linked with distinct types of diabetes can develop in parallel in a single person, including insulin resistance and autoimmune insulitis. |
Introduction
The classification of diabetes is not always straightforward due to the complexity of the mechanisms regulating glucose metabolism. The classification into type 1 diabetes (T1D) and type 2 diabetes (T2D), with the latter being by far the most common type of diabetes, is complemented by the monogenic forms of diabetes, secondary diabetes forms, and ‘latent autoimmune diabetes of the adults’ (LADA). The three diagnostic criteria conventionally used to diagnose LADA are non-specific and include the age at diagnosis, positivity for diabetes-associated autoantibodies (DAA), mostly for the antibody against glutamic acid decarboxylase (anti-GAD65), and the time to insulin requirement from diabetes diagnosis [1]. These criteria have evolved over time, and the most recent consensus comprises the following: age of > 30 years at diagnosis, DAA positivity and the absence of an insulin requirement for at least 6 months after diabetes diagnosis [2].
Adult-onset diabetes diagnosis and classification is usually based on clinical symptoms and signs, and DAA titres or C-peptide levels are not always investigated. Yet, establishing the correct diabetes type has implications for future management and prognosis and is therefore of high clinical importance. In this cross-sectional study, we aimed to establish the prevalence of DAA positivity (DAA +) in a group of individuals with a formal diagnosis of T2D who were referred to a tertiary diabetes centre in Slovakia within a predefined period of time. Given our clinical experience, we hypothesised that the prevalence of DAA positivity in this cohort would be higher than the reported rate of 4–14% from previously published studies [3,4,5,6,7,8,9,10]. We also hypothesised that not all DAA + individuals would meet the current diagnostic criteria for LADA. We took the advantage of the short inpatient stay of all participants included in this study, and, apart from performing a detailed anthropometric, biochemical, and clinical characterisation, we also collected data on hypoglycaemia incidence and severity during this period of time. These data then formed the basis for a detailed characterisation of DAA + individuals and their subsequent comparison with their DAA − counterparts in order to establish the characteristics linked with the presence of autoimmune insulitis. Taken together, the main purpose of the presented study was to establish the prevalence of DAA positivity in a group of individuals with a formal diagnosis of T2D who we referred to our diabetes centre within a given period of time and to perform a detailed characterisation and comparison of the DAA + individuals with the remaining DAA − patients from the cohort.
Methods
Study Design and Participants
This was a cross-sectional, observational study. All individuals with a formal diagnosis of T2D from all regions of Slovakia who were hospitalized at the National Institute of Endocrinology and Diabetology (NEDÚ), Ľubochňa, Slovakia between 1 January 2023 and 30 June 2023 for further evaluation and improvement of their diabetes treatment and its complications were included. This study was conducted in accordance with the ethical standards of the Helsinki Declaration of 1964 and its later amendments. The study protocol and all its procedures were reviewed and approved by the NEDÚ Ľubochňa and Jessenius Faculty of Medicine in Martin ethics committee. All participants signed a written informed consent prior to their inclusion in the study.
Study Procedures
On admission, data on age, sex, height, weight, body mass index (BMI), waist circumference, blood pressure (BP), age at the diagnosis of T2D and T2D duration, smoking status, glycated haemoglobin (HbA1c), fasting C-peptide levels, renal function, albuminuria, lipid profile, antidiabetic medication, type of insulin regimen and insulin dose, time from T2D diagnosis to insulin initiation, non-diabetic drug history, and presence and severity of diabetic complications and other comorbidities were collected. Data on hypoglycaemia (blood glucose ≤ 3.9 mmol/l) incidence, timing (nocturnal hypoglycaemia was defined as occurring between 22.00 and 06.00) and severity (severe hypoglycaemia was defined as an episode requiring external assistance for recovery) were collected during the 7-day period of the inpatient stay.
All participants had the following autoantibodies to pancreatic beta cell antigens tested: antibody against glutamic acid decarboxylase (anti-GAD65) was assayed through an enzyme-linked immunosorbent assay (ELISA) with 98% (n = 100) specificity and 92% (n = 50) sensitivity in the 2005 Diabetes Antibody Standardization Program (DASP) study [11] and with an assay cut-off value for positivity of ≥ 5 U/ml (DRG International Inc., Springfield, New Jersey, USA). Antibody against insulinoma-associated antigen IA-2 (IA-2A) was assayed through an ELISA with 98% (n = 90) specificity and 76% (n = 50) sensitivity and with an assay cut-off value for positivity of ≥ 7.5 U/ml (ElisaRSR™ IA-2 Ab version 2 manufactured by RSR Ltd., Cardiff, UK). Antibody against insulin (IAA) was assayed through an ELISA with 98.8% (n = 160) specificity and 71% (n = 100) sensitivity and with an assay cut-off value for positivity of ≥ 10 U/ml (ORGENTEC Diagnostika, Mainz, Germany). These three autoantibodies will be referred to as ‘diabetes-associated autoantibodies’ (DAA) in the remaining parts of this paper. A proportion of the participants were also tested for the presence of autoantibodies against thyroid peroxidase (TPO) (n = 350) and against thyroglobulin (n = 351). The TPO antibody was assayed through a competitive immunoassay with a cut-off value for positivity of ≥ 60 U/ml (ADVIA Centaur® XP/XPT anti-TPO manufactured by Siemens Healthcare Diagnostics, Munich, Germany). The antibody against thyroglobulin was assayed through a competitive immunoassay with 94.8% (n = 172) specificity and 98.5% (n = 65) sensitivity and with an assay cut-off value for positivity of ≥ 4.5 U/ml (ADVIA Centaur® XP/XPT Anti-Thyroglobulin II, manufactured by Siemens Healthcare Diagnostics, Munich, Germany).
Statistical Analysis
The data were collected on an Excel spreadsheet (version 2016, Microsoft Corp., Redmond, WA, USA) and further statistical analysis was performed with JASP (JASP team, 2022, version 0.16.2). Normally distributed data were reported as mean ± standard deviation (SD) (range), and data which did not follow a normal distribution were reported as median (range). The difference between independent samples was compared by Student’s t-test or the nonparametric Mann–Whitney U test. Frequency differences between groups were compared using the chi-square (χ2) test and Fisher’s exact test when appropriate. Linear correlations between variables were examined using the Spearman’s rank correlation coefficient rho, as these data did not follow a normal distribution. P < 0.05 was deemed statistically significant.
Results
Baseline Characteristics
Altogether, 692 individuals were included in the study. Baseline characteristics are shown in Table 1. Further participant characteristics, including the presence of comorbidities, use of non-diabetic medication and data on hypoglycaemia, are listed in Supplementary Table 1.
Positivity for Diabetes-Associated Autoantibodies
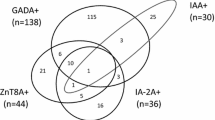
One hundred and thirty-six (136/692, 19.7%) individuals (91/136, 66.9% female) tested positive for anti-GAD65. Twenty-one (21/692, 3.0%) individuals (17/21, 81.0% female) tested positive for IA-2A. Twenty-eight individuals tested positive for IAA. Due to the fact that this assay cannot differentiate between autoantibodies against endogenous and exogenous insulin, those individuals who were on exogenous insulin therapy and were not positive for any of the two remaining DAA at the same time were not considered IAA/DAA positive in this study. Nineteen such individuals were identified, and these were excluded from subsequent analysis as one could not be certain of their true DAA status. Given these considerations, 9/692 (1.3%) individuals (4/9, 44.4% female) were deemed IAA positive in this study. Sixteen (16/692, 2.3%) individuals (15/16, 93.8% female) were double positive for anti-GAD65 and IA-2A, and 5/692 (0.7%) individuals (3/5, 60% female) were double positive for anti-GAD65 and IAA. Nobody was double positive for IAA and IA-2A, nor was there anyone who tested positive for all three autoantibodies. Taken together, 145/692 (21.0%) individuals (94/145, 64.8% female) tested positive for at least one of the three assayed DAA.
An autoantibody positivity that was 20 times above the cut-off value or higher (i.e. ≥ 100 U/ml for anti-GAD65, ≥ 150 U/ml for IA-2A and ≥ 200 U/ml for IAA) was considered strong positivity. Fifty-four (54/692, 7.8%) individuals (38/54, 70.4% female) were strongly positive for at least one out of the three DAAs. All 54 individuals were strongly positive for anti-GAD65 and 12 of these individuals (12/12, 100% female) were also strongly positive for IA-2A. Nobody in the studied population was strongly positive for IAA.
Comparison of Individuals with Autoantibody Positivity and Autoantibody Negativity
Next, we compared the DAA + individuals with the remaining DAA-negative (DAA −) participants. Comparisons in which statistically significant differences were detected are shown in Table 2. The DAA + individuals presented a higher proportion of females, a lower proportion of individuals with obesity, lower C-peptide levels and lower fasting triglycerides. Female (not male) DAA + individuals had higher HDL cholesterol levels in comparison to female DAA − participants. DAA + individuals experienced a higher number of hypoglycaemic events, and in the DAA + group there were more individuals who experienced at least one episode of hypoglycaemia during the 7-day inpatient stay. In relation to comorbidities, DAA + individuals had lower prevalences of arterial hypertension and ischaemic heart disease but a higher prevalence of autoimmune thyroiditis, which was associated with higher prevalences of positivity for thyroid peroxidase (TPO) autoantibodies and thyroglobulin autoantibodies. With regards to medication use, significantly fewer DAA + individuals were on metformin, fibrates, beta-blockers, and oral anticoagulants.
Correlations of Anti-GAD65 and Fasting C-Peptide Levels with Other Characteristics in Anti-GAD65-Positive Individuals
Linear correlations of anti-GAD65 titres and fasting C-peptide levels with relevant numerical variables were examined in the group of anti-GAD65-positive individuals. Anti-GAD65 titres showed the strongest (negative) correlation with the time to insulin initiation from diabetes diagnosis (Spearman’s rho − 0.508; 95% CI − 0.342, − 0.644; p < 0.001; n = 136) (Fig. 1). Correlations between anti-GAD65 titres and other numerical variables are listed in Supplementary Table 2. Fasting C-peptide levels showed the strongest (positive) correlation with the time to insulin initiation from diabetes diagnosis (Spearman’s rho 0.420; 95% CI 0.573, 0.239; p < 0.001; n = 136) (Fig. 2). Correlations between fasting C-peptide levels and other numerical variables are listed in Supplementary Table 3.
The Issue of Diabetes Type Re-classification
Of the 145 DAA + participants identified in this study, 137 were diagnosed with diabetes at an age of > 30 years, which is one of the current diagnostic criteria for LADA [2]. We aimed to examine how many of these individuals would meet the remaining third diagnostic criterion for LADA, namely the absence of an insulin requirement for at least 6 months after diabetes diagnosis. Data on the exact time of insulin initiation from diabetes diagnosis were available for 119/137 (86.7%) of them. Only 101/119 (84.9%) of these DAA + individuals aged over 30 years (66/101, 65.3% female) would meet the current diagnostic criteria for LADA, indicating that the remaining 18/119 (15.1%) individuals would not meet these criteria. These numbers imply that the prevalence of LADA in our cohort was 101/692 (14.6%). However, this diabetes type re-classification has limitations that are discussed in the next section of this paper.
Discussion
Several studies have examined the prevalence of DAA positivity in patients formally diagnosed with T2D, with reported prevalences in the range of 4–14% [3,4,5,6,7,8,9,10]. In our work, we report a much higher prevalence of DAA positivity, namely 21%, with anti-GAD65 positivity in 19.7% of all tested individuals. There are several possible explanations for this unusually high figure. First, the cohort of individuals included in our study does not represent the ‘general’ T2D population, and it also differs from the populations examined in the studies cited above. The participants included in this work (mostly) had diabetes of a longer duration, a rather high prevalence of diabetic complications and suboptimal glycaemic control. In the abovementioned studies, testing for DAA status usually took place at or around the time of diabetes diagnosis. It is therefore possible that in some individuals from our cohort, DAA positivity might have evolved at a later stage. Only two of the studies cited above, namely the LADA China study [5] and the UKPDS 25 study [6], reported a mean HbA1c value of around 9%, a figure similar to the one from our study. It has previously been reported that the prevalence of DAA positivity was higher in hospital settings in comparison to population-based studies [1]. Importantly, the prevalence of DAA positivity in a non-diabetic population is not zero. Positivity for DAA in a group of 383 non-diabetic individuals from western Finland was reported to be 4.4% [9]. It is possible that the prevalence of DAA positivity in the general Slovakian population is generally high, but this needs to be established in future studies. On the other hand, positivity for anti-GAD65 antibodies in non-diabetic individuals from eight European countries (Finland and Slovakia were not included) was reported to be 2.0% and did not differ much across the studied countries [12]. Lastly, apart from the true differences in prevalence, the wide range of reported DAA positivity prevalences could be partially explained by the use of different diagnostic tests with different cut-off values.
In line with previously published studies, the most frequently detected DAA in our study was the anti-GAD65 antibody, which is considered the most sensitive marker of autoimmune diabetes in adults [6, 13, 14]. The anti-GAD65 antibody was positive in 93.8% of all DAA + individuals in our study, a figure similar to that (90.5%) from the Action LADA 7 study [3].
We detected several anthropometric, biochemical, and clinical differences between DAA + and DAA − individuals. These data are largely in line with previously published studies, but there are some differences and novelties, too. It was found that DAA + individuals were leaner, had lower C-peptide and had a more favourable metabolic profile with lower fasting TAG and HDL-C levels in comparison to T2D patients without DAA [1, 2]. One study reported a higher proportion of women among DAA + T2D patients [3]. HDL-C levels in women are on average higher than in men throughout their lives, even after menopause [15]. For these reasons, we compared HDL-C levels separately for men and women. We showed that DAA + women had higher HDL-C levels in comparison to DAA − women, but no significant differences were observed in men, which is a novel finding. DAA + individuals have also been reported to have worse glycaemic control and to require insulin therapy more frequently and sooner after diabetes diagnosis [1, 2]. In our study, we did not detect any differences in HbA1c values and we observed a non-significant trend towards earlier insulin initiation (p = 0.059) in DAA + individuals. It is possible that this comparison would have reached statistical significance if a larger number of participants had been included. Of note, the proportion of insulin-using patients was generally high among both DAA + and DAA − individuals, which, in turn, can be explained by a longer diabetes duration and poor glycaemic control.
The data on hypoglycaemia are, to our knowledge, novel and will require further studies in the future, ideally with the use of continuous glucose monitoring (CGM) systems. In patients with insulin-treated T2D, low C-peptide levels are associated with greater glycaemic variability and a higher risk of hypoglycaemia [16]. Hence, a potential explanation for the increased incidence of hypoglycaemia and higher number of participants who experienced at least one episode of hypoglycaemia could be that DAA + participants had lower C-peptide levels and there were a significantly higher proportion of these participants with C-peptide values of < 0.2 nmol/l in comparison to DAA − individuals (data not shown). Lower use of metformin, fibrates and beta blockers in DAA + patients is likely to be linked with their leaner habitus, lower prevalences of arterial hypertension and ischaemic heart disease, and lower TAG levels, respectively.
The only study that examined linear correlations between anti-GAD65 titres and other continuous variables was the one by Radtke et al. from Finland, who reported a negative correlation between the anti-GAD65 titre and C-peptide levels (r = − 0.40, p = 0.009), but no significant correlation between the anti-GAD65 titre and time to insulin initiation from diagnosis could be detected (p = 0.07) [17]. In our study, on the contrary, the strongest (negative) correlation among the multitude of correlated variables was the one between the anti-GAD65 titre and time to insulin initiation from diagnosis (Spearman’s rho − 0.508, p < 0.001). The strength of correlation between anti-GAD65 titre and C-peptide levels in our study was similar to the one detected by the Finnish group (Spearman’s rho − 0.32, p < 0.001).
Only approximately 85% of DAA + individuals aged > 30 years at the time of diabetes diagnosis from our study would meet the current diagnostic criteria for LADA [2]. This diabetes re-classification has its limitations, though. First, the testing for DAA status had cross-sectional character and did not take place at or around the time of the diabetes diagnosis. We cannot be certain that these individuals tested positive for DAA at the time of diagnosis, and, conversely, the autoimmunity seen with DAA positivity could have evolved at a later time in some of DAA + individuals. A changing DAA status over time has been previously described in longitudinal studies [6, 18,19,20]. Secondly, the exact data on the time to insulin initiation from diabetes diagnosis were not available for all: only for 119/137 (86.7%) of eligible patients. Taking into account these considerations, approximately 15% of DAA + individuals aged over 30 years did not meet the current LADA diagnostic criteria. Yet, these individuals with a formal T2D diagnosis, mostly overweight or obese, show evidence for the presence of an autoimmune-mediated process against the pancreatic beta cells. We therefore conclude that in these individuals, adult-onset autoimmune diabetes with autoimmune insulitis and T2D, insulin resistance, and other features of metabolic syndrome are present at the same time. The timely and efficient diagnosis of LADA has clinical implications. The different natural history of LADA in comparison to T2D is often linked with worse glycaemic control and an earlier need for insulin therapy. The high prevalence of DAA positivity in our study underlines the need for more frequent anti-GAD65 screening in clinical practice. This call is in line with the recommendations of the International Expert Panel on Management of LADA in Adults stating that all newly diagnosed T2D patients should be screened for anti-GAD65 positivity in order to effectively identify patients with LADA [2]. In addition, we also recommend concomitant measurements of C-peptide levels, as these can inform on the further management of T2D and LADA, respectively.
The main strength of this study lies in the fact that it describes the prevalence of DAA positivity in a population of patients with a formal diagnosis of T2D of mostly longer duration and/or suboptimal glycaemic control. This is a single-centre study from a Central European country, and studies of this type, to the best of our knowledge, have not been published from this region so far. Our study thus adds data to the literature already available on this topic. Given the short inpatient stay of all study participants, we had the opportunity to formally assess the incidence and severity of hypoglycaemia. Our study also has several limitations. One of the main limitations is its cross-sectional character, which did not allow for any prospective follow-up. Our study protocol did not include measurements of fasting insulin levels, insulin and/or C-peptide response to oral glucose stimulation or genetic studies. Missing data on the exact time of insulin initiation in some of our patients precluded a more robust analysis. Lastly, we did not employ CGM technology to fully take advantage of the available CGM metrics, including testing for the prevalence of unrecognised hypoglycaemia, which was detected by the use of blinded CGM in people with T1D and T2D of longer duration [21, 22].
Conclusions
In this cross-sectional study from Slovakia, we report that approximately one in five individuals with a formal diagnosis of T2D showed signs of autoimmune insulitis, as evidenced by positivity for at least one of the three tested DAA (anti-GAD65, IAA or IA-2A). Individuals with DAA positivity differed from their DAA negative counterparts in several anthropometric, biochemical and clinical features. Some of these differences, like the higher incidence of hypoglycaemia, have not been described before. Only approximately 85% of DAA + individuals aged > 30 years at the time of diabetes diagnosis would meet the current diagnostic criteria for LADA, meaning that in the remaining 15% of these individuals, adult-onset autoimmune diabetes with autoimmune insulitis has evolved in parallel with T2D, insulin resistance and other features of metabolic syndrome. We conclude that more than one type of diabetes, according to current diagnostic criteria, might be present in a single person. These individuals in whom more than one type of diabetes coexist represent a unique population of people with diabetes that will require more detailed studies in the future.
References
Laugesen E, Ostergaard JA, Leslie RD, Danish Diabetes Academy Workshop and Workshop Speakers. Latent autoimmune diabetes of the adult: current knowledge and uncertainty. Diabet Med. 2015;32(7):843–52.
Buzzetti R, Tuomi T, Mauricio D, et al. Management of latent autoimmune diabetes in adults: a consensus statement from an international expert panel. Diabetes. 2020;69(10):2037–47.
Hawa MI, Kolb H, Schloot N, et al. Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: action LADA 7. Diabetes Care. 2013;36(4):908–13.
Maioli M, Pes GM, Delitala G, et al. Number of autoantibodies and HLA genotype, more than high titers of glutamic acid decarboxylase autoantibodies, predict insulin dependence in latent autoimmune diabetes of adults. Eur J Endocrinol. 2010;163(4):541–9.
Zhou Z, Xiang Y, Ji L, et al. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes. 2013;62(2):543–50.
Turner R, Stratton I, Horton V, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350(9087):1288–93.
Tiberti C, Giordano C, Locatelli M, et al. Identification of tyrosine phosphatase 2(256–760) construct as a new, sensitive marker for the detection of islet autoimmunity in type 2 diabetic patients: the non-insulin requiring autoimmune diabetes (NIRAD) study 2. Diabetes. 2008;57(5):1276–83.
Zinman B, Kahn SE, Haffner SM, et al. Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes. 2004;53(12):3193–200.
Tuomi T, Carlsson A, Li H, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48(1):150–7.
Zaharieva ET, Velikova TV, Tsakova AD, Kamenov ZA. Prevalence of positive diabetes-associated autoantibodies among type 2 diabetes and related metabolic and inflammatory differences in a sample of the Bulgarian population. J Diabetes Res. 2017;2017:9016148.
Torn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ, Participating L. Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia. 2008;51(5):846–52.
Rolandsson O, Hampe CS, Wennberg P, et al. Prevalence and regional distribution of autoantibodies against GAD65Ab in a European population without diabetes: the EPIC-interact study. Diabetes Care. 2015;38(8):e114–5.
Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes. 1993;42(2):359–62.
Fourlanos S, Dotta F, Greenbaum CJ, et al. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia. 2005;48(11):2206–12.
Legato MJ. Dyslipidemia, gender, and the role of high-density lipoprotein cholesterol: implications for therapy. Am J Cardiol. 2000;86(12A):15L-L18.
Christensen MB, Gaede P, Hommel E, Gotfredsen A, Norgaard K. Glycaemic variability and hypoglycaemia are associated with C-peptide levels in insulin-treated type 2 diabetes. Diabetes Metab. 2020;46(1):61–5.
Radtke MA, Midthjell K, Nilsen TI, Grill V. Heterogeneity of patients with latent autoimmune diabetes in adults: linkage to autoimmunity is apparent only in those with perceived need for insulin treatment: results from the Nord-Trondelag Health (HUNT) study. Diabetes Care. 2009;32(2):245–50.
Rasouli B, Grill V, Midthjell K, Ahlbom A, Andersson T, Carlsson S. Smoking is associated with reduced risk of autoimmune diabetes in adults contrasting with increased risk in overweight men with type 2 diabetes: a 22-year follow-up of the HUNT study. Diabetes Care. 2013;36(3):604–10.
Borg H, Gottsater A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and beta-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes. 2002;51(6):1754–62.
Desai M, Cull CA, Horton VA, et al. GAD autoantibodies and epitope reactivities persist after diagnosis in latent autoimmune diabetes in adults but do not predict disease progression: UKPDS 77. Diabetologia. 2007;50(10):2052–60.
Novodvorsky P, Bernjak A, Chow E, et al. Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Diabetes Care. 2017;40(5):655–62.
Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63(5):1738–47.
Acknowledgements
The authors would like to thank all the participants for their involvement in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The Rapid Service Fee was funded by the National Institute of Endocrinology and Diabetology (NEDÚ), Ľubochňa, Slovakia.
Author Contributions
Mariana Rončáková collected and analysed the data and reviewed the manuscript; Arash Davani, Veronika Mikušová and Ivana Ságová collected the data and reviewed the manuscript; Peter Novodvorský analysed the data and wrote the manuscript; Emil Martinka designed the study, reviewed the data and the manuscript. All authors contributed critical intellectual content, made important revisions to the manuscript and approved the final version of the manuscript for submission for publication. Emil Martinka is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
Parts of this study were presented at the 55th Annual Meeting of the European Association for the Study of Diabetes (EASD), Stockholm, Sweden, 19–23 September 2022.
Disclosures
Mariana Rončáková has served on speaker panels for Abbott, Berlin Chemie Menarini, Boehringer Ingelheim, Eli Lilly, Janssen, Novartis, Novo Nordisk, Medtronic, Merck, Sanofi and Roche and received travel grants from Sanofi, Novo Nordisk and Eli Lilly. Peter Novodvorský has served on speaker panels for Novo Nordisk, Eli Lilly, Sanofi, Boehringer Ingelheim, Abbott, Mundipharma and Krka; on advisory panels for Sanofi and Novartis; received honoraria or consulting fees from Merck, Boehringer Ingelheim and Eli Lilly; and received travel grants from Sanofi, Novo Nordisk, Eli Lilly and Berlin-Chemie Menarini. Emil Martinka has served on speaker panels for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Jansen, Johnson and Johnson, MSD, Medtronic, Novartis, Novo Nordisk, Organogenesis, Roche, Sanofi-Aventis, Servier, Takeda and Wörwag Pharma; on advisory panels for Abbott, Boehringer Ingelheim, Eli Lilly, MSD, Medtronic, Novo Nordisk, Sanofi-Aventis, Servier and Wörwag Pharma; received honoraria or consulting fees from Abbott, Boehringer Ingelheim, Eli Lilly, MSD, Medtronic, Novo Nordisk, Sanofi-Aventis, Servier and Wörwag Pharma. Arash Davani, Veronika Mikušová and Ivana Ságová declare no conflict of interest.
Compliance with Ethics Guidelines
This study was conducted in accordance with the ethical standards of the Helsinki Declaration of 1964 and its later amendments. The study protocol and all its procedures were reviewed and approved by the NEDÚ Ľubochňa and Jessenius Faculty of Medicine in Martin ethics committee. All participants signed a written informed consent prior to their inclusion in the study.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rončáková, M., Davani, A., Mikušová, V. et al. Prevalence of Positivity for Diabetes-Associated Autoantibodies in Individuals with Type 2 Diabetes and Their Further Characterisation: Cross-sectional Study from Slovakia. Diabetes Ther 14, 1537–1548 (2023). https://doi.org/10.1007/s13300-023-01440-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01440-2