Abstract
Introduction
The Chinese Diabetes Society recommends basal insulin and glucagon-like peptide-1 receptor agonists as an add-on therapy to first-line oral antihyperglycemic drugs for people with type 2 diabetes (T2D). Fixed-ratio combination of insulin glargine 100 U/ml (iGlar) and lixisenatide (iGlarLixi) is known to improve glycemic control in adults with T2D. However, the pharmacokinetics of iGlarLixi has not been evaluated in Chinese participants. The present study evaluated pharmacokinetics and safety of two iGlarLixi (10 U/10 μg and 30 U/15 μg) doses following single subcutaneous administration in healthy Chinese participants.
Methods
This was a Phase 1, single-center, open-label, parallel-group, randomized study in healthy Chinese adults who were randomized to receive a single dose of iGlarLixi with either 1:1 (10 U/10 μg) or 2:1 (30 U/15 μg) ratio of iGlar and lixisenatide. Primary objectives include assessment of pharmacokinetics of iGlar in iGlarLixi 30 U/15 μg group and the pharmacokinetics of lixisenatide in both the groups (iGlarLixi 10 U/10 μg and iGlarLixi 30 U/15 μg). Safety and tolerability were also assessed.
Results
In iGlarLixi 30 U/15 μg group, iGlar concentrations were low and quantifiable in three of ten participants, while its main metabolite (M1) was quantifiable in all participants, reflecting rapid conversion of iGlar to M1. Median INS-tmax was 14.00 h for iGlar and 13.00 h post-dose for M1. Absorption of lixisenatide was similar in both dose groups with median tmax of 3.25 and 2.00 h post-dose in both groups. The exposure increase was dose proportionate with a 1.5-fold increase in the lixisenatide dose. Adverse events observed were consistent with those previously reported with iGlar or lixisenatide.
Conclusion
iGlarLixi administration resulted in early absorption of both iGlar and lixisenatide with a good tolerability profile in healthy Chinese participants. These results are consistent with the previously published data from other geographic regions.
Trial registration
U1111-1194-9411.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
A combination of insulin glargine 100 U/ml (iGlar) and lixisenatide (iGlarLixi) improves the glycemic level through complimentary action and could offer an effective option for people with T2D |
There are no previously published data on the pharmacokinetic profile of iGlarLixi in Chinese participants. This study assessed the pharmacokinetics, safety and tolerability data for iGlarLixi following single subcutaneous administration in healthy Chinese adults |
What was learned from the study? |
After iGlarLixi 30 U/15 μg administration, iGlar rapidly converted to its active metabolite M1, which showed a median INS-tmax of 13.00 h. Lixisenatide showed early absorption with a median tmax of 3.25 and 2.00 h after administration of iGlarLixi 10 U/10 μg and iGlarLixi 30 U/15 μg, respectively |
Overall, single administration of iGlarLixi showed a good tolerability profile in healthy Chinese participants |
The results of this study are consistent with the previously published data from other geographic regions |
Introduction
China has the highest diabetes prevalence in the world, with approximately 141 million adults diagnosed with diabetes and about 1.4 million deaths due to diabetes or related complications reported in 2021 [1]. Postprandial hyperglycemia is a specific unmet need in Chinese people with T2D, highlighting the need for new treatment options which improve both fasting and postprandial plasma glucose (PPG). The Chinese Diabetes Society (CDS) guidelines recommend that, for people with suboptimally controlled T2D on first-line oral antihyperglycemic drugs (OADs), such as metformin, advancement options can include the addition of basal insulin (BI) or a glucagon-like peptide-1 receptor agonist (GLP-1 RA) [2]. iGlarLixi combines the complementary fasting plasma glucose (FPG) reduction of basal insulin glargine 100 U/ml (iGlar) and PPG reduction of the GLP-1 RA, lixisenatide (Lixi), in a convenient single daily injection, providing an efficacious treatment option for people advancing therapy from a variety of background therapies [3,4,5,6,7,8,9,10,11]. iGlarLixi has shown greater glycemic control than either iGlar or lixisenatide alone and has no additional risk of hypoglycemia compared to iGlar in people with T2D from Chinese mainland, Korea, Malaysia, Taiwan and Hong Kong SAR in the Asian Pacific region [12,13,14].
Studies assessing the pharmacokinetic profile of iGlar in healthy participants and people with type 1 diabetes (T1D) and T2D demonstrated a prolonged and slow absorption and a constant concentration/time profile over 24 h. The pharmacokinetic profile of iGlar was generally comparable when administered as a FRC or separate simultaneous injections of iGlar and lixisenatide [3]. The area under the plasma concentration versus time curve (AUC) of lixisenatide was comparable, regardless of whether it was administered alone or in combination with iGlar. Even though there was a decrease of 22%–34% in maximum plasma concentration (Cmax) of lixisenatide when administered as a FRC compared with separate simultaneous administration of iGlar and lixisenatide [3, 4], these pharmacokinetic differences were not considered clinically significant regarding the insulinotropic effect and sustained delay in gastric emptying reported with lixisenatide [15, 16].
To date, there are no published data on the pharmacokinetic profile of iGlarLixi in Chinese participants. Herein, we provide pharmacokinetics, safety and tolerability data for iGlarLixi following single subcutaneous administration in healthy Chinese adults.
Methods
Study Design
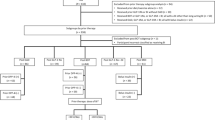
This was a Phase 1, single-center, open-label, parallel-group, randomized study in healthy Chinese adults (age range: 18–45 years) (World Health Organization universal trial number: U1111-1194-9411). Eligible participants with a body mass index (BMI) between 19.0 and 27.9 kg/m2 were randomized to receive a single dose of iGlarLixi with either 1:1 (iGlarLixi 10 U/10 μg) or 2:1 (iGlarLixi 30 U/15 μg) ratio of iGlar and lixisenatide (Fig. 1). Blood samples were collected to determine the plasma concentrations of iGlar and its metabolites (iGlarLixi 30U/15 μg group) and lixisenatide (both dose groups) for up to 24 h post dosing. Plasma samples for pharmacokinetic evaluation were collected at the following time points: for lixisenatide—at pre-dose and at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 10, 12 and 24 h post-dose; for iGlar—at pre-dose and at 0.5 and 1 h post-dose, and then at 2-h intervals from 2 to 24 h post-dose. The pharmacokinetic profile of iGlar was not assessed in the iGlarLixi 10 U/10 μg group, as almost all concentrations were below the lower limit of quantification (LLOQ, 0.2 ng/ml). The study was conducted in accordance with consensus ethics principles derived from international ethics guidelines, including the Declaration of Helsinki and the International Council for Harmonization (ICH) guidelines for Good Clinical Practice (GCP). The institutional review board of Beijing Hospital approved the study.
Study Objectives
The primary objectives of this study were to assess (1) pharmacokinetics of iGlar in iGlarLixi 30 U/15 μg group and (2) pharmacokinetics of lixisenatide in both groups (iGlarLixi 10 U/10 μg and iGlarLixi 30 U/15 μg). For iGlar and its metabolites, INS-Cmax, time to reach tmax (INS-tmax), AUC from time zero to 24 h (INS-AUC0–24) and the time to 50% of INS-AUC0–24 (t50%-INS-AUC0–24) were evaluated. For lixisenatide, Cmax, tmax, AUC, AUClast (AUC calculated using the trapezoidal method from time zero to time corresponding to the last observed concentration above the LLOQ) and apparent terminal half-life (t1/2z) were determined. The secondary objectives were to assess the safety and tolerability of iGlarLixi, including hypoglycemic events and treatment-emergent adverse events (TEAEs).
Pharmacokinetic Parameters
All the pharmacokinetic parameters like Cmax, Tmax, INS-AUC0–24, T50%-INS-AUC0–24, AUClast, AUC and t1/2z were calculated using noncompartmental methods from plasma lixisenatide and iGlar metabolite concentrations obtained after single dose administration with AUC = AUClast + Clast*t1/2z/0.693. For calculation of pharmacokinetic parameters, lixisenatide was assayed by ELISA (LLOQ is 5.5 pg/ml), and iGlar and its metabolites (M1 and M2) were assayed by LC-MS/MS (LLOQ = 0.200 ng/ml).
Statistical Analysis
Pharmacokinetic parameters of iGlar (INS-Cmax, INS-tmax, INS-AUC0-24 and t50%-INS-AUC0–24) and lixisenatide (Cmax, tmax, AUC, AUClast, t1/2z) are presented using arithmetic and geometric means, standard deviation (SD), coefficient of variation (CV%) and/or median (range) for each group. The pharmacokinetic analyses included all participants with no major or critical deviation related to investigational medicinal product and for whom pharmacokinetic data were considered sufficient and interpretable. Geometric means (90% confidence interval [CI]) were calculated for Cmax, AUC and AUClast of lixisenatide and for INS-AUC0–24. The distribution of tmax values is presented by histogram plots for each group. The distribution of t50%-INS-AUC0–24 values for iGlar is presented by treatment and by histogram plots for each group. Continuous variables (age, body weight and BMI) are presented using descriptive statistics for all participants.
Results
Participants
Twenty healthy Chinese participants were randomized to receive either iGlarLixi 10 U/10 μg (n = 10) or iGlarLixi 30 U/15 μg (n = 10). All participants completed the study. In the overall study population (N = 20), the mean age was 29.1 years, 60% were male, mean body weight was 66.0 kg, and the mean BMI was 23.6 kg/m2.
Pharmacokinetics
The iGlar main metabolite M1 was quantifiable in all participants, whereas iGlar concentrations above the LLOQ were observed in only three of ten participants. Subcutaneous administration of iGlarLixi 30 U/15 μg resulted in geometric mean (90% CI) INS-AUC0–24 of 3.50 (2.27, 5.39) ng h/ml for M1. Overall, iGlar concentrations were low, reflecting its rapid conversion to M1. The median INS-tmax was 14.00 h post-dose for iGlar and 13.00 h post-dose for M1, and the t50%- INS-AUC0-24 ranged from 10.88 to 14.63 h post-dose. Setting the concentration below the LLOQ to either 0 or half the LLOQ (0.1 ng/ml) provided similar results for M1 INS-AUC0–24. As only three samples in the iGlarLixi 10 U/10 μg group had quantifiable iGlar concentrations, INS-AUC0–24 was not determined. The iGlar metabolite M2 was not present in quantifiable concentrations in any of the samples (Table 1).
Absorption of lixisenatide appeared comparable between the two dose groups with median (range) tmax of 3.25 (1.00–5.00) and 2.00 (1.00–4.00) h post-dose in the iGlarLixi 10 U/10 μg and iGlarLixi 30 U/15 μg group, respectively. The mean estimate of t1/2z was approximately 2 h in both dose groups (Table 2). In the iGlarLixi 10 U/10 μg group, geometric mean (90% CI) exposure for lixisenatide in terms of Cmax, AUC and AUClast was 40.2 (34.5–46.9) pg/ml, 253.0 (209.1–305.6) pg h/ml and 226.2 (185.3–276.1) pg h/ml, respectively. In the iGlarLixi 30 U/15 μg group, geometric mean (90% CI) exposure values were 66.2 (56.7–77.2) pg/ml, 371.0 (306.7–448.0) pg h/ml and 342.0 (280.5–417.8) pg h/ml, respectively for Cmax, AUC and AUClast. The exposure increase was dose proportional: a 1.5-fold increase in the lixisenatide dose (from 10 to 15 μg) resulted in exposure ratios (90% CI) for Cmax, AUC and AUClast of 1.65 (1.33–2.05), 1.47 (1.12–1.92) and 1.51 (1.14–2.01), respectively (Fig. 2).
Safety
The observed TEAEs were consistent with the safety profiles of iGlar and lixisenatide. There were 17 TEAEs reporting by a total of 9 participants during the study. TEAEs were reported in five of ten (50%) participants in the iGlarLixi 10 U/10 μg group and four of ten (40%) participants in the iGlarLixi 30 U/15 μg groups; nausea and vomiting were the most frequently reported TEAEs. No dose-incidence relationship was observed. Among all TEAEs, seven of eight TEAEs were reported in the iGlarLixi 10 U/10 μg group (nausea, n = 2; vomiting, n = 2; dizziness, n = 1, injection site paresthesia, n = 1; injection site pruritus, n = 1 participant) and four of nine TEAEs reported in the iGlarLixi 30 U/15 μg group (nausea, vomiting, aspartate aminotransferase increased and decreased white blood cell count n = 1 each) were considered as related to the investigational medicinal product by the investigator. All TEAEs resolved spontaneously without sequelae and with no corrective treatment. There were no deaths, serious or severe TEAEs during the study.
Hypoglycemia
In the iGlarLixi 10 U/10 μg, hypoglycemia events were reported in five of ten (50%) participants, all within ≤ 2 h post iGlarLixi administration. One of these five participants also experienced hypoglycemia at 6 h and 12 h post iGlarLixi administration. In the iGlarLixi 30 U/15 μg group, hypoglycemia events were reported in eight of ten (80%) participants. Seven participants reported hypoglycemia within ≤ 3 h post iGlarLixi administration; three of them also reported hypoglycemia at 4 h, 8 h and 24 h, respectively, post iGlarLixi administration. One other participant in iGlarLixi 30 U/15 μg group reported hypoglycemia at 12 h post iGlarLixi administration. All hypoglycemia events were asymptomatic, with blood glucose level < 3.9 mmol/l and ≥ 3.0 mmol/l and resolved after administration of oral carbohydrates.
Discussion
In the present study in healthy Chinese participants, administration of iGlarLixi showed early absorption and systemic exposure expected for the two individual components, iGlar and lixisenatide, with a good tolerability profile. Compared to white populations, Asian individuals with T2D from China, Japan and South Korea have distinct physiology, such as greater insulin sensitivity, lower beta cell function and lower BMI [17, 18]. Along with these differences in standard medical practices, this distinct physiology contributes to a lower average effective insulin dose in Japanese participants versus white populations with T2D [19, 20]. Taking this into account, two ratios of iGlar to Lixi have been selected for development in China (10 U/10 μg and 30 U/15 μg) and were chosen for this study accordingly. The iGlarLixi 10 U/10 μg dose was selected to estimate the pharmacokinetics of a low dose. Concentrations of iGlar were not measured in the low-dose group as they were expected to be below the LLOQ in all samples. The iGlarLixi 30 U/15 μg dose enabled assessment of the full pharmacokinetic profile of lixisenatide but also that of iGlar or its metabolites. Plasma concentrations of iGlar and M1 were generally low, reaching a maximum of 0.24 ± 0.03 and 0.28 ± 0.09 ng/ml, respectively, in the iGlarLixi 30 U/15 μg group. The metabolite M1 was quantifiable in all participants, whereas iGlar concentrations above the LLOQ were observed in only three of ten participants in the iGlarLixi 30 U/15 μg group, indicating a rapid conversion of iGlar to M1. Similar observations showing rapid and nearly complete conversion of iGlar to its predominant metabolite, M1, have been reported in people with T1D after a single subcutaneous dose [21]. The pharmacokinetic profile of M1 in people with T1D showed maximum plasma concentrations approximately 12 h after the subcutaneous injection of iGlar [22], which is similar to the INS-tmax for M1 (13 h) observed in the present study. In a previous report, metabolic activity (AUC for glucose infusion rate from 0 to 30 h) was correlated with the plasma concentration profile of M1 (AUC0–30 h; r = 0.74; p < 0.001) [22]. Lucidi et al. also reported similar results, indicating that M1 accounted for most (> 90%) of the plasma insulin concentration and metabolic action in people with T2D after repeated daily subcutaneous dosing [23]. The other pharmacokinetic parameters of iGlar 30 U/15 μg (median INS-tmax: 14 h and INS-AUC0–24: 2.39 ng h/ml) reflect 24-h activity. Studies evaluating the pharmacokinetic and pharmacodynamics of iGlar in healthy participants or those with T1D from European countries showed a smooth stable pharmacokinetic and activity profile [24, 25]. Scholtz et al. studied the pharmacokinetics and glucodynamic variability with iGlar, NPH and ultralente insulin in 36 healthy participants which reported INS-tmax with iGlar was 12 h with less day-to-day 24-h variability compared with the other long-acting insulin [26].
In the present study, lixisenatide was rapidly absorbed (tmax of 2–3 h) with a dose proportional increase of 1.5-fold in Cmax and AUC. Raccah et al. reported results from a Phase 1 study evaluating pharmacokinetics of lixisenatide in participants aged ≥ 65 years and young healthy adult (< 65 years) participants. In that study, Cmax, tmax and AUC were 173 pg/ml, 1.75 h and 1060 pg h/ml, respectively, and the results confirmed the efficacy and safety of lixisenatide across age groups [27]. In a randomized, cross-over study conducted in white populations with T1D, the Cmax, tmax and AUC of lixisenatide were 47.3 pg/ml, 2.50 h and 213 pg h/ml, respectively, when iGlar and lixisenatide were administered as a premixed combination and 60.8 pg/ml, 1.75 h and 222 pg h/ml, respectively, for separate simultaneous injections. However, these observed differences in the pharmacokinetic profiles were not considered clinically relevant [28].
The TEAEs observed in the present study were consistent with previously reported safety profiles for iGlar or lixisenatide, and there were no new safety concerns. The most frequently reported TEAEs were gastrointestinal disorders, such as nausea and vomiting, which are commonly reported in previous studies with lixisenatide or iGlarLixi treatment [29, 30]. The TEAEs observed were of low severity (Grade 2 or lower), and all events resolved spontaneously without corrective treatment. Five participants in the iGlarLixi 10 U/10 μg group and eight in the iGlarLixi 30 U/15 μg group experienced hypoglycemia events, which may be expected with an insulin-containing treatment [31]. Notably, all hypoglycemia events were asymptomatic, and none had blood glucose < 3.0 mmol/l. Moreover, low risk of hypoglycemia with iGlarLixi was also reported by people with T2D in special conditions such as fasting during the Ramadan period [32].
Basal insulin therapy is often associated with risk of hypoglycemia, weight gain, challenges in reducing the post-prandial glucose (PPG) level and complexities with multiple injection and dose titration in Chinese people with T2D [33]. In people with T2D requiring BI and GLP-1 RA therapy, iGlarLixi could simultaneously provide an FPG-lowering effect of iGlar and the complementary PPG-lowering action of lixisenatide in a single injection. Moreover, lixisenatide is known to mitigate the weight gain associated with insulin therapy.
To our knowledge, this is the first study to report the pharmacokinetic profile and safety of iGlarLixi in healthy Chinese participants. However, this study included a small number of participants, and pharmacokinetic assessments were done after a single-dose administration of iGlarLixi. Another potential limitation is that the present study did not plan to evaluate ethnic differences. While the results appear to be similar to those previously reported in the white population, indirect comparison of data from studies with different populations and study designs should be interpreted with caution. Considering the phase I design of the present study, the efficacy and safety of iGlarLixi was not planned to be evaluated. However, in two phase III, randomized controlled trials conducted in participants from the Asia Pacific region, including Chinese mainland, Korea, Malaysia, Taiwan and Hong Kong SAR, iGlarLixi achieved significant HbA1c reductions, with no risk of hypoglycemia and body weight gain compared with iGlar and low levels of gastro-intestinal adverse events compared with lixisenatide in adults with T2D suboptimally controlled on oral agents or basal insulin [12, 13].
Conclusion
The pharmacokinetic profile of iGlar and lixisenatide following single subcutaneous administration of iGlarLixi in healthy Chinese adults was concordant with that previously reported for iGlar and lixisenatide individually. A single dose of iGlarLixi showed a safety and tolerability profile that was consistent with safety and tolerability profiles demonstrated in previous studies. These results are consistent with the previously published data from other geographic regions.
References
International Diabetes Federation: IDF Diabetes Atlas, 10th edition. Brussels, Belgium, International Diabetes Federation, 2021: https://diabetesatlas.org/. Accessed on March 01, 2022.
Diabetes Branch of Chinese Medical Association: Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2020 Edition). Chin J Endocrinol Metab. 2021;37:311–98.
Sanofi. Soliqua®: US prescribing information. 2019. Accessed March 2023. https://products.sanofi.us/soliqua100-33/soliqua100-33.pdf.
Sanofi. Suliqua®: EU summary of product characteristics. 2020. Accessed March 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/suliqua.
Blonde L, Anderson JE, Chava P, Dendy JA. Rationale for a titratable fixed-ratio co-formulation of a basal insulin analog and a glucagon-like peptide 1 receptor agonist in patients with type 2 diabetes. Curr Med Res Opin. 2019;35:793–804.
Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39:2026–35.
Terauchi Y, Nakama T, Spranger R, Amano A, Inoue T, Niemoeller E. Efficacy and safety of insulin glargine/lixisenatide fixed-ratio combination (iGlarLixi 1:1) in Japanese patients with type 2 diabetes mellitus inadequately controlled on oral antidiabetic drugs: a randomized, 26-week, open-label, multicenter study: the LixiLan JP-O2 randomized clinical trial. Diabetes Obes Metab. 2020;22:14–23.
Watada H, Takami A, Spranger R, Amano A, Hashimoto Y, Niemoeller E. Efficacy and safety of 1:1 fixed-ratio combination of insulin glargine and lixisenatide versus lixisenatide in Japanese patients with type 2 diabetes inadequately controlled on oral antidiabetic drugs: the LixiLan JP-O1 randomized clinical trial. Diabetes Care. 2020;43:1249–57.
Blonde L, Rosenstock J, Del Prato S, et al. Switching to iGlarLixi versus continuing daily or weekly GLP-1 RA in type 2 diabetes inadequately controlled by GLP-1 RA and oral antihyperglycemic therapy: the LixiLan-G randomized clinical trial. Diabetes Care. 2019;42:2108–16.
Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39:1972–80.
Kaneto H, Takami A, Spranger R, Amano A, Watanabe D, Niemoeller E. Efficacy and safety of insulin glargine/lixisenatide fixedratio combination (iGlarLixi) in Japanese patients with type 2 diabetes mellitus inadequately controlled on basal insulin and oral antidiabetic drugs: the LixiLan JP-L randomized clinical trial. Diabetes Obes Metab. 2020;22:3–13.
Yang W, Dong X, Li Q, et al. Efficacy and safety benefits of iGlarLixi versus insulin glargine 100 U/mL or lixisenatide in Asian Pacific people with suboptimally controlled type 2 diabetes on oral agents: The LixiLan-O-AP randomized controlled trial. Diabetes Obes Metab. 2022;24(8):1522–33.
Yuan X, Guo X, Zhang J, et al. Improved glycaemic control and weight benefit with iGlarLixi versus insulin glargine 100 U/mL in Chinese people with type 2 diabetes advancing their therapy from basal insulin plus oral antihyperglycaemic drugs: results from the LixiLan-L-CN randomized controlled trial. Diabetes Obes Metab. 2022;24(11):2182–91.
Feng W, Wang W, Meng R, Wu G, et al. Lixisenatide is effective and safe as add-on treatment to basal insulin in Asian individuals with type 2 diabetes and different body mass indices: a pooled analysis of data from the GetGoal Studies. BMJ Open Diabetes Res Care. 2021;9: e002290.
Lorenz M, Pfeiffer C, Steinstraesser A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes–relationship to postprandial glycemia. Regul Pept. 2013;185:1–8.
Becker RH, Stechl J, Steinstraesser A, et al. Lixisenatide reduces postprandial hyperglycaemia via gastrostatic and insulinotropic effects. Diabetes Metab Res Rev. 2015;31:610–8.
Saisho Y. Obesity, type 2 diabetes and beta cell failure: an Asian perspective. J Mol Genet Med. 2014;s1:008.
Cho YM. Characteristics of the pathophysiology of type 2 diabetes in Asians. Ann Laparosc Endosc Surg. 2017;2:14.
Odawara M, Ohtani T, Kadowaki T. Dosing of insulin glargine to achieve the treatment target in Japanese type 2 diabetes on a basal supported oral therapy regimen in real life: ALOHA study subanalysis. Diabetes Technol Ther. 2012;14:635–43.
Meneghini LF, Mauricio D, Orsi E, et al. The Diabetes Unmet Need with Basal Insulin Evaluation (DUNE) study in type 2 diabetes: achieving HbA1c targets with basal insulin in a real-world setting. Diabetes Obes Metab. 2019;21:1429–36.
Shiramoto M, Eto T, Irie S, et al. Single-dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab. 2015;17(3):254–60.
Bolli GB, Hahn AD, Schmidt R, et al. Plasma exposure to insulin glargine and its metabolites M1 and M2 after subcutaneous injection of therapeutic and supratherapeutic doses of glargine in subjects with type 1 diabetes. Diabetes Care. 2012;35(12):2626–30.
Lucidi P, Porcellati F, Rossetti P, et al. Metabolism of insulin glargine after repeated daily subcutaneous injections in subjects with type 2 diabetes. Diabetes Care. 2012;35(12):2647–9.
Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49(12):2142–8.
Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644–9.
Scholtz HE, Pretorius SG, Wessels DH, et al. Pharmacokinetic and glucodynamic variability: assessment of insulin glargine, NPH insulin and insulin ultralente in healthy volunteers using a euglycaemic clamp technique. Diabetologia. 2005;48(10):1988–95.
Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31(2):204–11.
European Medicines Assessment Report https://www.ema.europa.eu/en/documents/assessment-report/suliqua-epar-public-assessment-report_en.pdf. Accessed 21 Dec 2022.
Hinnen D, Strong J. iGlarLixi: A new once-daily fixed-ratio combination of basal insulin glargine and lixisenatide for the management of type 2 diabetes. Diabetes Spectr. 2018;31(2):145–54.
Terauchi Y, Nakama T, Spranger R, et al. Efficacy and safety of insulin glargine/lixisenatide fixed-ratio combination (iGlarLixi 1:1) in Japanese patients with type 2 diabetes mellitus inadequately controlled on oral antidiabetic drugs: a randomized, 26-week, open-label, multicentre study: the LixiLan JP-O2 randomized clinical trial. Diabetes Obes Metab. 2020;22(Suppl 4):14–23.
Rodbard HW, Gough S, Lane W, et al. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized begin trials. Endocr Pract. 2014;20(4):285–92.
Hassanein M, Malek R, Shaltout I, et al. Real-world safety and effectiveness of iGlarLixi in people with type 2 diabetes who fast during Ramadan: The SoliRam observational study. Diabetes Metab Syndr. 2023;17: 102707.
Inoue M, Lorenz M, Muto H, et al. Effect of a single dose of insulin glargine/lixisenatide fixed ratio combination (iGlarLixi) on postprandial glucodynamic response in Japanese patients with type 2 diabetes mellitus: A phase I randomized trial. Diabetes Obes Metab. 2019;21(8):2001–5.
Acknowledgements
The authors are grateful to all study participants and would like to thank all the trial staff and investigators who participated in data collection for the study.
Funding
Sponsorship for this study and the Rapid Service Fee for this publication was funded by Sanofi.
Medical Writing and Editorial Assistance
Coordination for the development of this manuscript and assistance with the revisions were provided by Ana Merino-Trigo, Sanofi. The authors acknowledge Silpi Mishra, MPharm, and Deepak Reddy Gade, PhD, of Sanofi, for their contribution to the manuscript development and coordination.
Author Contributions
Conception and design of the work: Aixin Shi, Mengmeng Shuai, Wolfgang Schmider, Alex Jiang and Na Yang. Study conduct and data acquisition: Panpan Xie, Aixin Shi, Xuemei He and Xin Gao. Analysis of data: Mengmeng Shuai, Wolfgang Schmider, Alex Jiang and Na Yang. All authors contributed to the data interpretation, drafting, critical review, and revision of the manuscript and approved the final version for submission.
Disclosures
Panpan Xie, Aixin Shi, Xuemei He and Xin Gao have nothing to disclose. Mengmeng Shuai, Wolfgang Schmider, Alex Jiang, and Na Yang are employees of Sanofi and may hold company stock/shares.
Compliance with Ethics Guidelines
The study was conducted in accordance with consensus ethics principles derived from international ethics guidelines, including the Declaration of Helsinki, and the International Council for Harmonization (ICH) guidelines for Good Clinical Practice (GCP). The Institutional review board of Beijing Hospital has approved the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Xie, P., He, X., Gao, X. et al. Pharmacokinetics and Safety of iGlarLixi in Healthy Chinese Participants: Results of a Phase 1 Randomized Study. Diabetes Ther 14, 1387–1397 (2023). https://doi.org/10.1007/s13300-023-01434-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01434-0