Abstract
Introduction
Effective and scalable solutions to support management of Type 2 Diabetes (T2D) at a distance are a priority for health systems worldwide. The use of personalised care planning has been shown to be effective at improving the health outcomes and the experience of care amongst people with T2D and other long-term health conditions. Here we describe a specific example of such an intervention.
Methods
The sample comprised 197 participants with T2D randomised to either the active intervention group with digital health planning (App + usual care), with 115 participants, or the control group (usual care), with 82 participants. We analysed data in relation to changes in body mass index (BMI) and glycated haemoglobin (HbA1c) over a 6-month follow-up period. We also analysed responses to questionnaires sent out and held interviews with participants that were in the active treatment group and therefore had a care plan created and access to an app.
Results
The active treatment group had significant reductions in HbA1c (p < 0.01) and BMI (p < 0.037) vs the control group (no significant change). The average percentage change in HbA1c for the treatment group over 6 months was − 7.4% (± SE 1.4%), compared with 1.8% (± SE 2.1%) for the control group. The average percentage change in BMI for the treatment group was − 0.7% (± SE 0.4%) and it was − 0.2% (± SE 0.5%) for the control group. A higher percentage of the active treatment group reduced their HbA1c and BMI than the control group. For HbA1c, 72.4% of the active treatment group reduced their HbA1c, compared to 41.5% of the control group. For BMI, 52.7% of the active treatment group experienced a reduction, compared to 42.9% for the control group. Self-measured quality of life (QoL) improved for patients in the active treatment group, shown by an increase in their pre-trial to post-trial EQ-5D-5L rating by an average of 0.0464 (± SE 0.0625), compared to a decrease of 0.0086 (± SE 0.0530) for the control group. The average EQ VAS score also increased pre- to post-trial for the active treatment group, on average by 8.2%, whereas it decreased by an average of − 2.8% for the control group.
Conclusion
These findings point to how the provision of personalised plans of care, support and education linked to a mobile app, can result in HbA1c and BMI reduction for many individuals with T2D. The use of a patient management app as well as a personalised care plan also led to an improvement in patient self-rated QoL and engagement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The number of people with type 2 diabetes (T2D) is steadily increasing, thus there is a need for them to self-manage more effectively to reduce the complications of this disorder and reduce the pressure within primary care of treating these patients. |
This study set out to evaluate whether a personalised care planning software and patient-facing mobile app could improve health outcomes amongst people with T2D. |
The provision of personalised plans of care, support and education linked to a mobile app, resulted in HbA1c and BMI reductions over a 6-month period for individuals with T2D. |
The provision of personalised plans of care, support and education linked to a mobile app, resulted in an improvement in quality of life and patient engagement for individuals with T2D. |
Introduction
The increasing number of people living with long-term conditions (LTCs) is one of the biggest challenges facing our health and social care systems. In 2019, 463 million adults globally were living with diabetes, and this number is predicted to increase to 700 million by 2045 [1, 2], with type 2 diabetes (T2D) making up 90% of this population. This high prevalence results in T2D costing the UK National Health Service (NHS) over £14 billion per year [3] with T2D accounting for an estimated 15–25% of all appointments at a local surgery level, depending on the practice. Not only this, but T2D is also difficult to manage because of the multiple lifestyle and self-management behaviours required to effectively manage this disease and avoid complications [4], which then result in even higher costs for the NHS. The broader social determinants of health make behaviour change for people with T2D even more challenging [2].
For these reasons, effective and scalable solutions to support management of T2D at a distance are a priority for health systems worldwide [5]. The COVID-19 pandemic has resulted in many health systems looking to digital health solutions to increase access and self-management support [6], with more than 500 million people now using mobile apps to support their management of health conditions [5]. Not only this, but the use of personalised care planning has been shown to be effective at improving the health outcomes and the experience of care amongst people with T2D and other LTCs [7, 8].
It has been recognised for many years that achievement of a target glycated haemoglobin (HbA1c) level for people with T2D is associated with a reduced risk of cardiovascular events, as well as a reduced mortality rate [9]. There are significant financial, organisational, technological and time barriers amongst multidisciplinary teams (MDTs) of healthcare professionals (HCPs) in primary care that make it hard to co-create integrated plans of care, support and education for people with T2D, consequently impacting outcomes [10]. Furthermore, the Healum Collaborative Care Planning Software and App was designed to reduce these barriers and provide patients with co-created care and support plans to help them self-manage their LTC better.
This prospective randomised control trial (RCT) was designed to evaluate whether personalised care planning software and a patient-facing mobile app could improve health outcomes amongst patients with T2D through the delivery of personalised plans of care, support and education to allow patients to self-manage their diabetes more effectively, all accessible on a mobile device.
- Primary outcome:
-
Does the Healum app improve health outcomes (HbA1c and BMI) and patient quality of life measures amongst patients with T2D over a 6-month period?
- Secondary outcome:
-
Does the Healum app improve the patient’s engagement levels measured by their capability, opportunity and/or motivation to change their behaviour?
Methods
RCT Participants
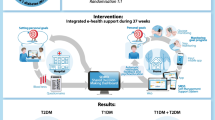
This prospective RCT compared baseline and 6-month clinical data from 197 people (Fig. 1) with T2D across three surgeries in the south of the UK plus 10 surgeries in Eastern Cheshire (UK). In this data set, the participant age range was 22–85 years. Out of a total of 197 participants, 116 (58.9%) were male, 65 (33.0%) were female and 16 (8.1%) did not report their gender, classed as ‘other’. The inclusion criteria for the RCT were that participants had to be over the age of 18 and diagnosed with T2D. The sample size was based on a power calculation which estimated a requirement to screen 450 patients to give 390 patients participating, at a power of 83.6% to see a difference between intervention + usual care group (p < 0.05).
The control group consisted of 21 women (25.6%), 50 men (61.0%) and 11 ‘other’ (13.4%), and the active treatment group had 44 women (38.3%), 66 men (57.4%) and 5 ‘other’ (4.3%) (Table 1). The average age for the control group was 65.2 years and the age range was 34–85 years. For the active treatment group, the average age was 61.1 years and the age range was 22–85 years (Table 1).
Inclusion Criteria
Participants who:
-
Are capable of reading the patient information sheet and giving informed consent themselves
-
Are over the age of 18
-
Are living with type 2 diabetes mellitus
-
Have a smartphone and are able to use three apps that are not categorised as utilities (i.e. clock, calculator, phone, etc.)
-
Have an HbA1c of over 58 mmol/mol
Exclusion Criteria
Participants who:
-
Are pregnant
-
Are unable to read or speak English, owing to the fact that the content of the app is only available in English at this time
-
Are in another research study that clashes with the burdens of this study or where the tasks of any other study combined with this study are deemed too burdensome for the individual, as determined by the lead investigator at each GP practice and the chief investigator of this study.
Design
People with T2D with HbA1c greater than 58 mmol/mol (7.5%) were randomised to either the active intervention group (usual care + app) or control group (usual care) (Fig. 1). The research considers patients who were diagnosed with T2D as a long-term condition (i.e. not new patients). The intervention group received a co-created personalised care plan which involved daily lifestyle prompts and a range of recommended resources including educational content and self-management tools, as well as addressing patient objectives and concerns. Randomisation did not influence other decisions about diabetes management. Therefore all patients received the standard diabetes care they normally would from their GP (usual care), with active treatment participants receiving a co-created care plan and app access on top of this. The clinical data in this report pertains to the health markers body mass index (BMI) and blood glucose as measured by HbA1c. Quality of life and patient activation were determined quantitatively. The HCPs of the GP surgeries who would go on to use the software and app with patients received an hour training session, the cost of this training was not directly remunerated.
Data Collection
Owing to the Healum software’s interoperability with EMIS Web (an electronic patient record system used by the majority of GPs in primary care), patient profiles can be instantly created in Care Planning Healum directly through EMIS, whereby all necessary patient information is extracted. This integration between EMIS Web and the Healum software allowed direct extraction of health outcomes data (timestamped latest and previous test results) from the EMIS system which an evaluator was able to analyse to determine the impact of the intervention on health outcomes. All patients opened in the Healum software had a randomised 16 digit user ID created for them and all their app usage metrics, including engagement with each specific feature, were tracked against this ID. The evaluator was then able to look at each user individually to see their app usage patterns as well as their health outcomes data. Joe Stock of Kaleidoscope Consultants is responsible for the protection of sensitive personal data of patients involved in the RCT.
Health Outcomes Analysis
To analyse the health outcomes for the active treatment group in comparison to the control group, we looked at those patients that had their most recent BMI and HbA1c test results reported at least 90 days after their first GP appointment during the RCT. This was tracked as the first time the patient’s profile was opened by an HCP in the Healum care planning software. This ensured that any changes in BMI and HbA1c per patient that took place before the RCT, and consequently before the participant received any intervention, were not included in the data analysis. Changes in BMI and HbA1c results during the trial were calculated for each patient; this value was used to then calculate the average percentage change over a 6-month period for both BMI and HbA1c, to allow for more comparable data across patients. Therefore, all health results included in this report are the average change in BMI/HbA1c over 6 months. App engagement was quantified in terms of goals joined, markers tracked, resources viewed, time spent within the app and the number of app sessions per user and was analysed in comparison to health outcome data to determine the impact of using the Healum app more frequently or using different features of the app.
Quality of Life Analysis
Health-related quality of life (QoL) was self-rated by participants using the online survey “Healum Diabetes Study Survey” which included the National Institute for Health and Care Excellence (NICE)-validated EQ-5D-5L questionnaire [11]. Participants were sent a link by text message from their GP practice to complete this survey at two time points, once at the beginning of their time on the trial, and again after 6 months on the trial. The responses were extracted directly from the platform and then each patient’s responses were matched together to allow a comparison of their self-rated QoL before and after their 6 months on the trial, either using the app (active treatment group) or not (control group).
The survey involved:
-
1.
EQ-5D-5L questionnaire [11] which is a widely used generic measure of health status consisting of two parts, the EQ-5D-5L descriptive system and accompanying EQ visual analogue scale (EQ VAS).
The descriptive system comprises five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression, with each dimension having five levels. The five levels are as follows: no problems, slight problems, moderate problems, severe problems and extreme problems. The patient selected statement results in a one-digit number that expresses the level selected for that dimension. A summary index with a maximum score of 1 can be derived from these five dimensions by conversion with a table of scores. The maximum score of 1 indicates the best health state, by contrast with the scores of individual questions, where higher scores indicate more severe or frequent problems. The EQ VAS records the patient’s self-rated health on a vertical visual analogue scale, where the endpoints are labelled ‘The best health you can imagine’ and ‘The worst health you can imagine’. EQ VAS can be used as a quantitative measure of health outcomes that reflect the patient’s own judgement of their health.
-
2.
Questions relating to patients’ engagement with their health. These open-ended questions were designed to assess the knowledge, skills and confidence of patients to manage their health.
The responses to the patient engagement questions were grouped into responses: “Yes” (Y); “Somewhat Yes” (SY); “Neutral” (Ne); “Somewhat No” (SN) and “No” (N), whereby an answer of “Yes” indicated higher patient engagement in their health.
Statistical Methods
In the prospective RCT, the primary outcome measure was change in HbA1c and BMI from baseline to 6 months after the participant joined the trial. Analysis was restricted to those with their latest measurements at least 90 days after their first appointment as part of the trial. Baseline characteristics of all eligible participants and the analysis population were reported. We calculated the mean change in HbA1c and BMI during the trial relative to the baseline HbA1c/BMI value for each patient. All results are reported as mean and standard error (SE). Student’s t test was used to determine the level of significance of difference.
Compliance with Ethics Guidelines
This study gained approval from the Greater Manchester West Ethics Committee on 18 June 2020 (IRAS ID 272569). The study was performed in accordance with the Declaration of Helsinki 1964 and its later amendments. This was not a collaborative research project, the delivery of the research was carried out exclusively by Healum Ltd. All participants gave informed consent in relation to participation in the study and were aware that their anonymised data would be analysed and included in peer review publications.
Results
Participant Characteristics
In this prospective RCT, the treatment group (App + usual care) and control (usual care) groups were made up of 115 and 82 participants (Fig. 1).
Although the study design specified health outcomes data to be collected at month 0 and month 6 of the trial, as a result of pressure within primary care as a result of the COVID-19 pandemic, the participating patients had a range of times between their original and second health outcomes tests; their second HbA1c ranged from 134 to 418 days (median 188) after their first. For those in the active treatment group, their second HbA1c ranged from 156 to 532 days (median 201) after their first check.
Changes in Health Outcomes (HbA1c and BMI)
The average starting BMI for the members of the control group who fitted the inclusion criteria was 31.1 kg/m2 (± 1.0 kg/m2) and for members of the active treatment group it was 31.2 kg/m2 (± 0.7 kg/m2) (Table 1). For the treatment group, the latest BMI mean dropped to 30.8 kg/m2 (± SE 0.7 kg/m2), with the control group’s latest BMI average decreasing to 30.9 kg/m2 (± SE 0.7 kg/m2) (Table 1).
The mean average starting HbA1c for members of the control group who fitted the inclusion criteria was 68.9 mmol/mol (± SE 1.7 mmol/mol) and that of the active treatment group was 70.6 mmol/mol (± SE 1.6 mmol/mol) (Table 1). For the treatment group, the end HbA1c result decreased to a mean average of 64.1 mmol/mol (± SE 1.4 mmol/mol), with the control group end HbA1c result increasing to an average of 69.1 mmol/mol (± SE 1.8 mmol/mol) (Table 1).
A higher proportion of the active treatment group experienced a reduction in their BMI and HbA1c than the control group. For BMI, 52.7% of the active treatment group had a reduction, compared to 42.9% for the control group (Fig. 2b). For HbA1c, 72.4% of the active treatment group reduced their HbA1c, compared to 41.5% of the control group (Fig. 2a).
The active treatment group had significant reductions in HbA1c (p < 0.01) and BMI (p < 0.037) vs the control group (no significant change). The mean average percentage change in BMI for the active treatment group was − 0.7% (± SE 0.4%) and it was − 0.2% (± SE 0.5%) for the control group (Fig. 3). The average percentage change in HbA1c for the active treatment group over 6 months was − 7.4% (± SE 1.4%), but 1.8% (± SE 2.1%) for the control group (Fig. 3).
Higher engagement with the app, as measured by the number of app sessions a participant completed, led to better health outcomes for users. Of all the users who used the app within the first month after download, the group that used it at least two times had an average BMI change of − 0.6%, compared to 0.2% for those that used it just once. This trend was also shown with HbA1c, where those that used it at least two times had an average change of − 8.5% compared to − 1.5% for those that used it just once. For those users still using the app during days 91–180 after app download, the users that used the app at least 10 times within that time period had the best HbA1c outcomes. The average HbA1c change was − 12.3% for the group of users with at least 10 sessions, compared to 0.2% for those that used it just once.
Changes in Quality of Life and Patient Engagement
The results from the questionnaires completed by participants showed an improvement in their self-rated QoL. We were able to match pre- and post-trial survey responses for participants in the control group and participants in the active treatment group. Patients in the active treatment group demonstrated an improvement in their self-measured QoL score over the course of the trial, shown by an increase in their EQ-5D-5L rating by an average of 0.0464 (Fig. 4a). Whereas, for those in the control group, the QoL decreased, shown by their EQ-5D-5L rating decreasing on average by 0.0086 (Fig. 4a). Patients in the active treatment group also demonstrated an improvement in their self-measured standard of their health shown by their EQ VAS score increasing by 8.2% on average (Fig. 4b). For those patients in the control group, their EQ VAS score changed on average by − 2.8% indicating a reduction in patients’ self-measured standard of their health (Fig. 4b).
Effect of Medication Changes on RCT Results
During the study, 12 patients (10.4%) in the active treatment group and 17 patients (20.5%) in the control group had a change in their prescribed diabetes medication as part of their standard care provided by their HCP. Specifically focusing only on those changes to either a glucagon-like peptide 1 (GLP-1) agonist or insulin, seven individuals switched to either a GLP-1 agonist or insulin: five patients switched to a GLP-1 agonist and two switched to insulin (one to Semglee and one to Tresiba).
Discussion
In this prospective RCT, the findings point to how the provision of personalised plans of care, support and education linked to a mobile app, resulted in HbA1c and BMI reductions over a 6-month period for individuals with T2D, over a control (usual care) group. The use of a patient management app as well as a personalised care plan also led to an improvement in patient self-rated QoL and patient engagement over a control group (as measured by EQ-5D-5L and EQ VAS). These findings are relevant to clinical practice as this was a real-world trial conducted in primary care, whereby practice nurses delivered the intervention in an everyday practice setting without requiring additional resources or specialist courses.
Comparison with Previous Studies and Evidence
Suboptimal glycaemic control in patients with T2D is a critical risk factor in development of diabetes complications [12]; therefore, solutions that can improve blood glucose control are of high importance. Our findings are in keeping with other studies that have examined how app-based solutions may improve glycaemic control when used as adjuncts to usual care [13,14,15,16]. A 2017 meta-analysis of 13 studies on mobile apps for diabetes suggested overall efficacy in reducing HbA1c, with a mean decrease in intervention compared with control of 0.44%, as well as increased perception of self-care among mobile app users [17]. Two RCTs into mobile apps (BlueStar and Monica) with comprehensive features for self-management of T2D, similar to the Healum App, demonstrated reductions in HbA1c.
One study investigated whether a digital health intervention could improve self-management of T2D [18]. The program focuses on supporting sustainable dietary changes, primarily around carbohydrate restriction by utilising behaviour change techniques, including goal setting, peer support, and behavioural self-monitoring, as well as personalised downloadable resources, including recipes and meal plans tailored to ethnicity, weekly shopping budget, and dietary preferences. This digital app, prescribed to adults with T2D and prediabetes in a primary care setting supporting a transition to a low-carbohydrate diet, was shown to be effective in improving glycaemic control and enabling weight loss [18]. This study supports our findings that self-management and behaviour change support can aid patients in improving their glycaemic control.
Two studies involving patients at risk of diabetes, rather than already diagnosed, the Alive-PD (NutritionQuest) [19] behavioural change intervention and the Mobile Diabetes Prevention Program (mDPP) [20], demonstrated the use of mobile apps to promote lifestyle modifications, with both interventions demonstrating efficacy in reducing body weight.
These other digital health interventions, which have been previously investigated, focus on similar principles to the Healum Collaborative Care Planning Software and App, and therefore support the proposal that offering self-management and behaviour change tools to patients with T2D can improve their clinical outcomes.
The World Health Organization (WHO) explains that medication concordance is the extent to which the patient agrees to follow their HCP’s recommendations including medication regimen, dietary and lifestyle changes [21]. Studies have shown there is a causal relationship between the number of hospitalisations and medication adherence by individuals with T2D [12]. Hence, it is evident that ensuring that people following their medication regimen, dietary and lifestyle recommendations is a critical factor in diabetes management. Therefore, several attempts have been made to find solutions to improve concordance [12]. Thus, this trial is relevant as it looked at whether a digital care plan including dietary and lifestyle recommendations could improve adherence and ultimately health outcomes in people with T2DM.
A strength of this research conducted is the fact that it was a real-world study, where practice nurses delivered the intervention in an everyday practice setting, which is rare for digital health studies in T2D [22]. The sample size was based on a power calculation which estimated a requirement to screen 450 patients to give 390 patients participating, at a power of 83.6% to see a difference between intervention + usual care group (p < 0.05). We were not able to achieve this target because of prevailing circumstances, specifically the COVID-19 pandemic. However, despite the lower recruitment number, we still saw a statistically significant difference between the active treatment and control groups. Finally longer-term follow-up is required and is planned, to determine whether the effects on HbA1c seen here are enduring.
Conclusion
These findings point to how the provision of personalised plans of care, support and education linked to a mobile app, can result in HbA1c and BMI reduction over a median 6-month period for many individuals with T2D. The use of a patient management app as well as a personalised care plan led to an improvement in patient self-rated QoL and engagement. This has the potential to lead to longer-term health benefits.
References
Unnikrishnan R, Pradeepa R, Joshi SR, Mohan V. Type 2 diabetes: demystifying the global epidemic. Diabetes. 2017;66(6):1432–42.
International Diabetes Federation. IDF Diabetes Atlas 9th edition 2019. https://www.diabetesatlas.org. Accessed 1 Mar 2023.
Diabetes.co.uk. Cost of diabetes 2019. https://www.diabetes.co.uk/cost-of-diabetes.html. Accessed 1 Mar 2023.
Hartmann-Boyce J, Morris E, Goyder C, et al. Diabetes and COVID-19: risks, management, and learnings from other national disasters. Diabetes Care. 2020;43(8):1695–703.
Gonzalez JS, Tanenbaum ML, Commissariat PV. Psychosocial factors in medication adherence and diabetes self-management: implications for research and practice. Am Psychol. 2016;71(7):539–51.
Quinn LM, Davies MJ, Hadjiconstantinou M. Virtual consultations and the role of technology during the COVID-19 pandemic for people with type 2 diabetes: the UK perspective. J Med Internet Res. 2020;22(8):e21609.
Velardo C, Shah SA, Gibson O, et al. Digital health system for personalised COPD long-term management. BMC Med Inform Decis Mak. 2017;17:19.
Foley P, Steinberg D, Levine E, et al. Track: a randomized controlled trial of a digital health obesity treatment intervention for medically vulnerable primary care patients. Contemp Clin Trials. 2016;48:12–20.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12.
Fisher L, Polonsky WH, Hessler D. Addressing diabetes distress in clinical care: a practical guide. Diabet Med. 2019;36:803–12.
The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
Shrivastava TP, Goswami S, Gupta R, Goyal RK. Mobile app interventions to improve medication adherence among type 2 diabetes mellitus patients: a systematic review of clinical trials. J Diabet Sci Technol. 2023;17(2):458–66.
Bennett GG, Steinberg D, Askew S, et al. Effectiveness of an app and provider counseling for obesity treatment in primary care. Am J Prev Med. 2018;55:777–86.
Salari R, Niakan R, Kalhori S, et al. Mobile-based and cloud-based system for self-management of people with type 2 diabetes: development and usability evaluation. J Med Internet Res. 2021;23:e18167.
Larsen K, Akindele B, Head H, et al. Developing a user-centered digital clinical decision support app for evidence-based medication recommendations for type 2 diabetes mellitus: prototype user testing and validation study. JMIR Hum Factors. 2022;9:e3347.
Zimmermann G, Venkatesan A, Rawlings K, Scahill MD. Improved glycemic control with a digital health intervention in adults with type 2 diabetes: retrospective study. JMIR Diabetes. 2021;6:e28033.
Shan R, Sarkar S, Martin SS. Digital health technology and mobile devices for the management of diabetes mellitus: state of the art. Diabetologia. 2019;62(6):877–87. https://doi.org/10.1007/s00125-019-4864-7.
Summers C, Tobin S, Unwin D. Evaluation of the Low Carb Program digital intervention to instigate and sustain self-management of type 2 diabetes and prediabetes at an NHS England GP clinic: a single-arm prospective study. JMIR Diabetes. 2020. https://doi.org/10.2196/25751.
Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10):e240. https://doi.org/10.2196/jmir.4897.
Fukuoka Y, Gay CL, Joiner KL, Vittinghoff E. A novel diabetes prevention intervention using a mobile app: a randomized controlled trial with overweight adults at risk. Am J Prev Med. 2015;49(2):223–37. https://doi.org/10.1016/J.AMEPRE.2015.01.003.
Sabaté E. Adherence to long term therapies: evidence for action. Defining adherence. World Health Organization. 2003:3–5. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. Accessed 4 Feb 2021.
Baradez C, Liska J, Brulle-Wohlhueter C, Pushkarna D, Baxter M, Piette J. Brief digital solutions in behavior change interventions for type 2 diabetes mellitus: a literature review. Diabetes Ther. 2022. https://doi.org/10.1007/s13300-022-01244-w.
Acknowledgements
We thank the participants of the study and Vernova Healthcare CIC for supporting the delivery of the project. We thank the Greater Manchester West Ethics Committee for providing ethics approval for this study.
Funding
This study was funded by Innovate UK (Grant Number 27744). The funding for publication of the study results, including the Rapid Service fee and the Open Access fee, was provided by the original Innovate UK grant and Healum Ltd.
Medical Writing and Editorial Assistance
There was no medical writing assistance for this manuscript.
Author Contributions
Dr Adrian H Heald conceived the study with Jonathan Abraham and co-led on writing this paper. Sarah Roberts led on the writing of this paper and assisted with statistical analysis. Lucia Albeldo Gimeno provided invaluable input in relation to coordination of the study, recruitment and writing this paper. Erin Gillingham, Morwenna James, Alison White and Laura Beresford delivered the study in Eastern Cheshire, supported patient recruitment and contributed to the final manuscript. Anuj Saboo led on technical development and implementation. Alan Crofts led on statistical analysis. Jonathan Abraham conceived the study with Adrian H Heald, led on the sourcing of funding and reviewed the final version of the manuscript.
Disclosures
Dr Adrian H Heald, Sarah Roberts, Lucia Albeldo Gimeno, Erin Gillingham, Morwenna James, Alison White, Laura Beresford, Anuj Saboo, Alan Crofts and Jonathan Abraham have nothing to disclose.
Compliance with Ethics Guidelines
This study gained approval from the Greater Manchester West Ethics Committee on 18 June 2020 (IRAS ID 272569). The study has been performed in accordance with the Helsinki Declaration of 1964 and its later amendments. This was not a collaborative research project, the delivery of the research was carried out exclusively by Healum Ltd. All participants gave informed consent in relation to participation in the study and were aware that their anonymised data would be analysed and included in peer review publications.
Data Availability
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Heald, A.H., Roberts, S., Albeda Gimeno, L. et al. A Randomised Control Trial to Explore the Impact and Efficacy of the Healum Collaborative Care Planning Software and App on Condition Management in the Type 2 Diabetes Mellitus Population in NHS Primary Care. Diabetes Ther 14, 977–988 (2023). https://doi.org/10.1007/s13300-023-01404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01404-6