Abstract
Introduction
Many Indians are at high risk of type 2 diabetes mellitus (T2DM). The blood glucose level can be improved through a healthy lifestyle (such as physical activity and a healthy diet). Yoga can help in T2DM prevention, being a culturally appropriate approach to improving lifestyle. We developed the Yoga Programme for T2DM Prevention (YOGA-DP), a 24-week structured lifestyle education and exercise (Yoga) program that included 27 group Yoga sessions and self-practice of Yoga at home. In this study, the feasibility of undertaking a definitive randomized controlled trial (RCT) was explored that will evaluate the intervention’s effectiveness among high-risk individuals in India.
Methods
A multicenter, two-arm, parallel-group, feasibility RCT was conducted in India. The outcome assessors and data analysts were blinded. Adults with a fasting blood glucose level of 100–125 mg/dL (i.e., at high risk of T2DM) were eligible. Participants were randomized centrally using a computer-generated randomization schedule. In the intervention group, participants received YOGA-DP. In the control group, participants received enhanced standard care.
Results
In this feasibility trial, the recruitment of participants took 4 months (from May to September 2019). We screened 711 people and assessed 160 for eligibility. Sixty-five participants (33 in the intervention group and 32 in the control group) were randomized, and 57 (88%) participants were followed up for 6 months (32 in the intervention group and 25 in the control group). In the intervention group, the group Yoga sessions were continuously attended by 32 (97%) participants (median (interquartile range, IQR) number of sessions attended = 27 (3)). In the intervention group, Yoga was self-practiced at home by 30 (91%) participants (median (IQR) number of days per week and minutes per day self-practiced = 2 (2) and 35 (15), respectively). In the control group, one (3%) participant attended external Yoga sessions (on Pranayama) for 1 week during the feasibility trial period. There was no serious adverse event.
Conclusions
The participant recruitment and follow-up and adherence to the intervention were promising in this feasibility study. In the control group, the potential contamination was low. Therefore, it should be feasible to undertake a definitive RCT in the future that will evaluate YOGA-DP’s effectiveness among high-risk people in India.
Feasibility Trial Registration
Clinical Trials Registry—India (CTRI) CTRI/2019/05/018893; registered on May 1, 2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Many Indians are at high risk for type 2 diabetes mellitus. The blood glucose level can be improved through a healthy lifestyle (such as physical activity and a healthy diet). Yoga can help in type 2 diabetes mellitus prevention, being a culturally appropriate approach to improving lifestyle. |
A multicenter, two-arm, parallel-group, feasibility randomized controlled trial was conducted in India. The outcome assessors and data analysts were blinded. Adults at high risk for type 2 diabetes mellitus were eligible. In the intervention group, participants received a Yoga program to prevent type 2 diabetes mellitus. In the control group, participants received enhanced standard care. |
The participant recruitment and follow-up and adherence to the intervention were promising in this feasibility study. In the control group, the potential contamination was low. Therefore, it should be feasible to undertake a definitive randomized controlled trial in the future that will evaluate the effectiveness of the intervention among high-risk individuals in India. |
Introduction
Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder with significant health and socioeconomic consequences, and India has the second-largest T2DM population in the world [1]. An individual at high risk of T2DM has a higher blood glucose level than normal but lower than the level for T2DM, and more than 77 million people in India are at high risk of T2DM [2]. The chances of developing T2DM and its complications are higher among these people compared to individuals with a normal blood glucose level [3]. Indians rapidly develop T2DM from the high-risk state and have one of the highest rates across many ethnicities [4]. The major risk factors for T2DM are physical inactivity and an unhealthy diet, i.e., an unhealthy lifestyle [3]. A cost-effective and sustainable strategy is to screen individuals at high risk of T2DM and provide a lifestyle intervention that is effective [3, 5, 6]. Effective lifestyle interventions can improve their blood glucose level and can have other health benefits [7,8,9]. However, Indians usually have a low physical activity level, and they usually consume an unhealthy diet [10,11,12].
Yoga, an ancient mind–body discipline, originated in the Indian subcontinent and incorporates physical activity and a healthy diet [13]. Various styles of Yoga are practiced, but one style is not necessarily superior or more authentic than another, and all focus on the same important topic, i.e., a healthy lifestyle [14]. In general, Yoga’s acceptability is high in India as it fits people’s health beliefs and culture [15, 16]. A gentle approach is used in Yoga, and it is safe and easy to learn, requires minimal guidance and maintenance costs, and can be practiced indoors as well as outdoors [15]. People who are old or with comorbidities can practice it [14, 15]. Yoga includes low-intensity and moderate-intensity practices (< 3.5 kcal/min and 3.5–7.0 kcal/min, respectively) [14, 17,18,19]. In addition, it is an activity that strengthens the muscles [14]. Therefore, Yoga can contribute to the goal of routine lifestyle advice which is given to individuals at high risk of T2DM to prevent it.
Yoga’s mechanism of action in T2DM and related conditions has been reported before [20]. Briefly, its benefits on T2DM-related risk profiles seem to occur predominantly through the following pathways: (1) by decreasing the activation and reactivity of the sympathoadrenal system and the hypothalamic–pituitary–adrenal axis, and by fostering feelings of well-being, it may lessen the effects of stress and promote multiple beneficial downstream effects on the neuroendocrine status, metabolic function, and related systemic inflammatory responses; (2) by directly stimulating the vagus nerve, it may improve the parasympathetic activity and lead to beneficial changes in the cardiovagal function, energy state, mood, and related neuroendocrine, metabolic, and inflammatory responses. Furthermore, Yoga may reduce body mass index (BMI), and the reduction in BMI lowers the risk of T2DM [3].
Effectiveness systematic reviews suggest the benefits and safety of Yoga in T2DM and related conditions [21,22,24]. However, the duration of most of the primary studies was short (≤ 3 months), and these studies often had significant methodological limitations. In addition, the intervention development process was not always reported, and some did not describe the intervention in detail. Even if they did, the interventions were heterogeneous. Therefore, robustly designed studies are needed to evaluate the utility of Yoga for preventing T2DM among high-risk individuals. We systematically developed the Yoga Programme for T2DM Prevention (YOGA-DP) [25]. Our aim is to conduct a definitive randomized controlled trial (RCT) in the future that will evaluate YOGA-DP’s effectiveness among high-risk individuals in India compared to enhanced standard care. The chances of successful completion of the definitive RCT will improve if the feasibility of its key elements is tested before commencement [26, 27]. Therefore, the feasibility of undertaking the definitive RCT was explored in this study.
Methods
The feasibility study protocol is published elsewhere [28].
Study Design
A multicenter, two-arm, parallel-group, feasibility RCT was conducted. The outcome assessors and data analysts were blinded.
Study Setting
We conducted this feasibility study at two Yoga centers in India, namely Bapu Nature Cure Hospital and Yogashram (BNCHY, New Delhi) in north India and Swami Vivekananda Yoga Anusandhana Samsthana (S-VYASA, Bengaluru) in south India. These centers are accessed by individuals from a range of socioeconomic backgrounds. We used three languages (English, Hindi, and Kannada) in conducting this feasibility study.
Sample Size
In a feasibility trial, a formal sample size estimation is not usually needed [29]. It is recommended to recruit at least 50 participants in a feasibility trial [30]. Thus, we recruited a total of 65 participants in this feasibility trial, after taking into consideration the loss to follow-up.
Screening and Recruitment Strategies
The following strategies were used to inform people about this feasibility study: posters were placed and pamphlets were distributed at several locations (such as in these Yoga centers, health clinics, communities, parks, and religious places) and door-to-door visits were conducted in several communities at different times of the day. Screening camps were organized at several places (such as in these Yoga centers, communities, and religious places) for identifying potential participants. After giving the participant information sheet to potential participants, describing this feasibility study to them, and answering their questions, we requested that those interested in this feasibility trial provide written informed consent. The screening was conducted after receiving written informed consent, i.e., the fasting blood glucose level was assessed using a glucometer (by finger-prick; using either HemoCue Glucose 201+ System or Accu-Chek Active) [31, 32]. People with a fasting blood glucose level of 100–125 mg/dL (i.e., potentially at high risk of T2DM) [33] were requested to visit the Yoga center for eligibility assessment, including a confirmatory venous blood test using the standardized glucose oxidase–peroxidase method. This eligibility assessment was conducted after receiving further written informed consent. Blood samples collected were sent immediately to the accredited laboratories for analysis within an hour. If this was not possible for any reason, the serum was immediately separated by centrifugation and stored in a − 80 °C freezer for analysis on the next day.
Eligibility Criteria
People aged 18–74 years, with a fasting blood glucose level of 100–125 mg/dL (i.e., at high risk of T2DM) [33], and who were safe to do physical activities (checked using the Physical Activity Readiness Questionnaire (PAR-Q)/clinician) [34], willing and able to attend the intervention/control sessions on their own, and able to provide written informed consent were included in this feasibility study. The following people were excluded: pregnant women, those with glycated hemoglobin (HbA1c) ≥ 6.5% (i.e., with T2DM [33]; venous blood test using the high-performance liquid chromatography method) or a serious or uncontrolled medical condition (e.g., cancer), or those who regularly practiced Yoga (i.e., ≥ 150 min/week) or were receiving (or had plans to receive during the feasibility trial period) any related non-pharmaceutical/pharmaceutical intervention (e.g., glucose-lowering medication).
Randomization
A computer-generated randomization schedule was used to randomize eligible participants to the intervention or control group (1:1, block randomization, stratified by sex and site). Sequential random sampling (140/group) was used, with 35 equal blocks generated using STATA V.15. The block size was four and fixed. This central randomization was performed by an independent statistician, based at the Centre for Chronic Disease Control (CCDC), New Delhi, India, and the allocation was accessed by the recruiting site staff by telephone call. There was an exception to this rule to avoid contamination, i.e., people recruited from the same household or who were close relatives or friends were randomized to the same group. Baseline data were collected after randomization. Participants and intervention/control providers were not blinded to group allocation, but the outcome assessors and data analysts were blinded to the feasibility trial assignment.
Interventions
Intervention (YOGA-DP)
Intervention details are published elsewhere [25]. Briefly, YOGA-DP was a 24-week structured lifestyle education and exercise (Yoga) program and included 27 group Yoga sessions and self-practice of Yoga at home using the program booklet and a video. The intervention was delivered by YOGA-DP instructors, qualified and experienced Yoga teachers with formal training received on the program. The program included Shithilikarana Vyayama (loosening exercises), Surya Namaskar (sun salutation exercises), Asana (Yogic poses), Pranayama (breathing practices), and Dhyana (meditation) and relaxation practices. Female instructors were available for women. Group sessions were delivered locally (e.g., at these Yoga centers and community centers). Participants were able to join at their convenience as these sessions were run at different time points of the day (including evening and weekend sessions). We reimbursed participants’ local travel costs to attend these sessions. We also invited a family member or someone close to the participant to join him/her in these sessions. After completing the program, participants were strongly encouraged to use the intervention materials and maintain a healthy lifestyle in the long term.
We ensured intervention fidelity, and YOGA-DP instructors were regularly trained on the basis of an instructor manual. In addition, we regularly observed and evaluated these sessions using a checklist to ensure delivery according to the manual. Structured and instructive feedback was provided to them to improve their performance.
Control (Enhanced Standard Care)
No formal T2DM prevention program is available in India, although some healthcare professionals offer rudimentary advice. Therefore, in the control group, participants received the routine lifestyle advice to prevent T2DM among them in the form of a leaflet, provided by another team member (i.e., different from the YOGA-DP instructor) to avoid contamination.
Study Parameters and Data Collection
We estimated the essential parameters needed for designing the definitive RCT, e.g., participant recruitment and follow-up (for 6 months), adherence to the intervention, potential contamination in the control group, and standard deviations (SDs) of the outcomes. Details are provided in the feasibility study protocol [28]. Briefly, the following outcomes were assessed: biochemical parameters (fasting blood glucose, HbA1c, total cholesterol, high-density lipoprotein, low-density lipoprotein, very low-density lipoprotein, and triglyceride), physiological parameters (systolic blood pressure, diastolic blood pressure, and heart rate), anthropometric parameters (weight, BMI, and waist circumference), lifestyle (diet, physical activity (the International Physical Activity Questionnaire (IPAQ)-Short was used and categorized into low and moderate/high and vigorous, moderate, walking, and sitting) [35], tobacco usage, and alcohol consumption), health-related quality-of-life (the EuroQol-5D (EQ-5D) dimensions were categorized into no (level 1; no problems) and yes (level 2 to 5; problems)) [36], depression, anxiety, and stress (the Depression, Anxiety and Stress Scale (DASS) dimensions were categorized into normal and mild/moderate/severe/extremely severe) [37], and self-efficacy (for assessing confidence in participant’s ability to practice Yoga) [38].
Data Analyses and Reporting
For categorical data, numbers and percentages were calculated. For continuous data, summary measures of mean or median and spread were calculated. In terms of the percentage of loss to follow-up and withdrawal, the two groups were compared using the chi-squared test. Being a feasibility trial, it was not powered to find a difference in trial outcomes at 6 months between the two trial arms. However, unadjusted mean difference (MD) or odds ratio (OR) with a 95% confidence interval (CI) were reported to indicate initial estimates of effects. Subsequently, we conducted the analysis of covariance (ANCOVA) for four critical outcomes (fasting blood glucose, HbA1c, BMI, and waist circumference), and regression coefficient and 95% CI were reported. In model 1, the respective baseline value was adjusted for; in model 2, the respective baseline value and age were adjusted for. The analysis was based on the intention-to-treat principle. There was no plan to conduct an interim analysis. STATA V.15 was used to analyze the data. The extension of the Consolidated Standards of Reporting Trials (CONSORT) statement for randomized pilot and feasibility trials was used to report the results [39].
Ethics and Related Issues
The study was conducted in accordance with the Declaration of Helsinki. The research ethics committees of the following institutes gave ethics approval: Faculty of Medicine and Health Sciences, University of Nottingham (UK), CCDC (India), BNCHY (India), and S-VYASA (India). This feasibility study was performed in accordance with relevant guidelines and regulations. We obtained written informed consent from participants. This feasibility trial was registered with the Clinical Trials Registry—India (CTRI) (CTRI/2019/05/018893; registered on May 1, 2019). India’s Health Ministry’s Screening Committee (HMSC) also approved this feasibility study. The independent Trial Steering Committee (TSC) monitored and supervised this feasibility study.
Serious Adverse Events
We planned to collect information on serious adverse events (including hospitalization for at least 24 h and mortality) occurring in the feasibility trial participants that might be attributed to the interventions. An independent clinician was to adjudicate the association of any such event to the interventions on the basis of medical and scientific judgment.
Participant Withdrawal
We planned to withdraw participants from the feasibility trial on the basis of their request or at the discretion of the site investigator, e.g., in case the participant was no longer safe to do physical activities (determined by PAR-Q/clinician) [34] or was diagnosed with diabetes (and would receive the standard treatment).
Results
Recruitment and Follow-up
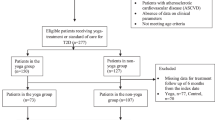
In this feasibility trial, participants were recruited from 18 May 2019 to 19 September 2019 (i.e., when the first person was approached to participate and the last participant was randomized, respectively). It took 4 months and 2 days to recruit participants. We approached 727 people to participate. Of these, 711 were screened before eligibility assessment and 160 were assessed for eligibility. Sixty-five participants (33 in the intervention group and 32 in the control group) were randomized, and this excludes deregistered participants who did not meet the inclusion criteria but were recruited or who were recruited late. Five participants were randomized to the same group as the first participant (as either they were from the same household or were close relatives or friends). Fifty-seven (88%) participants were followed up for 6 months (32 in the intervention group and 25 in the control group). There was one (3%) loss to follow-up in the intervention group and six (19%) loss to follow-up in the control group, and the difference was statistically significant (p = 0.04). There was no withdrawal in the intervention group and one (3%) withdrawal in the control group, but the difference was statistically insignificant (p = 0.31) (see Fig. 1 CONSORT flowchart for details). As a result of the COVID-19 lockdown in India, the follow-up of one intervention group participant was delayed and limited information was collected over the telephone from two intervention group participants and one control group participant.
Intervention Adherence
In this feasibility study, the group Yoga sessions were continuously attended by 32 (97%) participants, and one participant discontinued because of hospitalization for a few hours for vomiting, high blood pressure, and chest pain (this was not counted as a serious adverse event). The median (interquartile range, IQR) number of group Yoga sessions attended was 27 (3). Yoga was self-practiced at home by 30 (91%) participants, and the median (IQR) number of days per week and minutes per day self-practiced was 2 (2) and 35 (15), respectively.
Potential Contamination in the Control Group
One (3%) participant attended external Yoga sessions (on Pranayama) for 1 week during the feasibility trial period (self-reported). Yoga classes were available outside, but YOGA-DP was not available externally.
Outcomes
Table 1 reports the baseline characteristics of the feasibility trial participants. The mean (± SD) age of participants was 42.1 (7.7) years, and 25 (38%) were female. The mean (± SD) fasting blood glucose was 110.3 (8.3) mg/dL, HbA1c was 5.9 (0.3) %, BMI was 27.6 (5) kg/m2, and waist circumference was 93 (13.2) cm. At baseline, the two groups were mostly similar except for a few variables, which could be due to the small sample size.
Table 2 reports the unadjusted outcomes at 6 months.
Biochemical, physiological, and anthropometric parameters—The fasting blood glucose, HbA1c, weight, BMI, and waist circumference were lower in the intervention group compared to the control, but the differences were statistically insignificant. The high-density lipoprotein was higher in the intervention group compared to the control, but the difference was statistically insignificant.
Lifestyle—The percentage of participants reporting no/occasional intake of high-fat/deep-fried food was higher in the intervention group compared to the control. The moderate physical activity time was higher in the intervention group compared to the control, but the difference was statistically insignificant. Sitting time was lower in the intervention group compared to the control, but the difference was statistically insignificant. The percentage of participants reporting no tobacco usage was higher in the intervention group compared to the control. In addition, the odds of tobacco usage were lower in the intervention group compared to the control, but the difference was statistically insignificant.
Health-related quality-of-life—The percentage of participants reporting no problems in all five dimensions of EQ-5D was higher in the intervention group compared to the control. In addition, the odds of problems in the three dimensions (mobility problems, problems in taking self-care, and problems in doing usual activities) were lower in the intervention group compared to the control, but the differences were statistically insignificant. The visual analog scale score was higher in the intervention group compared to the control, but the difference was statistically insignificant.
Depression, anxiety, and stress—The percentage of participants reporting no problems in all three dimensions of DASS was higher in the intervention group compared to the control.
Self-efficacy in Yoga practice—The self-efficacy was higher in the intervention group compared to the control, but the difference was statistically insignificant.
Table 3 reports the adjusted four critical outcomes at 6 months.
Fasting blood glucose, HbA1c, BMI, and waist circumference—After adjustment for the respective baseline value (model 1) and baseline value and age (model 2), BMI was found to be lower in the intervention group compared to the control, and the difference was statistically significant (regression coefficient − 0.56; 95% CI − 1.00 to − 0.11 and regression coefficient − 0.56; 95% CI − 1.02 to − 0.11, respectively). In addition, fasting blood glucose, HbA1c, and waist circumference were lower, but the differences were statistically insignificant.
Serious Adverse Events
There was no serious adverse event.
Discussion
We conducted a multicenter feasibility RCT in India to assess the feasibility of undertaking the definitive RCT in the future that will evaluate YOGA-DP’s effectiveness among high-risk individuals in India. The participant recruitment and follow-up and adherence to the intervention were promising in this feasibility study. There was low potential contamination in the control group. Compared to other T2DM prevention RCTs in India and globally [27, 40], the recruitment in this feasibility trial was without any major hurdles. In this feasibility study, the dropout rate was 12% which is consistent with the findings of a systematic review (less than 15–20% in most RCTs on Yoga interventions) [41]. The only follow-up-related challenge we faced was towards the end of this feasibility trial, and this was due to the COVID-19 lockdown in India. Therefore, it should be feasible to undertake a definitive RCT. This feasibility study provided estimates of important parameters needed for designing the definitive RCT.
In the definitive RCT, the first co-primary outcome will be the incidence of T2DM, indicated by either a fasting blood glucose level of at least 126 mg/dL or an HbA1c level of at least 6.5% at 1-year follow-up [3]. Repeat testing will be conducted in asymptomatic participants to confirm the diagnosis [3], and they will only be included as achieving the co-primary outcome if repeat testing of either fasting blood glucose or HbA1c is above the cutoff. The second co-primary outcome will be BMI at 1-year follow-up [42]. The reduction in BMI lowers the risk of T2DM [3]. It should be noted that compared to other ethnicities, South Asians are at higher risk of T2DM at equivalent BMI levels [43]. We expect that the cumulative incidence of T2DM over 1 year will be 18% in the control group [44, 45]. In the intervention group, we will be interested in a 50% reduction in the cumulative incidence of T2DM [46, 47]. Therefore, in the definitive RCT, we will need to recruit at least 356 participants per group (a total of 712), with 90% power and assuming significance (alpha; two-tailed) of 2.5% to conservatively allow for co-primary outcomes. This sample size will be sufficient for the other primary outcome, considering the mean (± SD) BMI at the baseline was 27.6 (5) kg/m2 in this feasibility trial and our interest in at least a 5% reduction in BMI at 1 year in the intervention group [42, 48].
Initial estimates (as this feasibility trial was not powered for effectiveness) show some beneficial effects of the intervention on many critical outcomes, including fasting blood glucose, HbA1c, BMI, and waist circumference. In the adjusted models, BMI was lower in the intervention group compared to the control and the difference was statistically significant. No serious adverse events occurred. Similar beneficial effects and safety of Yoga on T2DM-related outcomes have been synthesized in several effectiveness systematic reviews [21,22,24]. In the future, the adequately powered definitive RCT will be able to provide a clear answer.
In the definitive RCT, if YOGA-DP is found to be effective, it will be a need-sensitive and evidence-based intervention for preventing T2DM among high-risk individuals, not only in India but also globally. Yoga’s popularity is not restricted to India and other South Asian countries, and it is increasingly becoming popular in other nations [49, 50]. T2DM and its associated costs are global concerns, and a low-cost intervention to prevent T2DM will be of worldwide interest. More evidence-based choices will be available to people for preventing T2DM. The future burden of T2DM (such as clinical, personal, and economic) on patients, families, health systems, and economies will be prevented. The prevention of T2DM may extend to the prevention of its complications. Individuals will become healthier overall, and the intervention may empower people at the same time for managing their health. The intervention has become even more relevant during the COVID-19 pandemic, as it can be delivered online or in-person (outdoors or indoors by following the social distancing rules), and Yoga can be self-practiced at home.
Semi-structured qualitative interviews (as part of the qualitative process evaluation) were conducted with feasibility trial non-participants, participants, YOGA-DP instructors, and staff to explore trial- and intervention-related barriers and facilitators [28, 51, 52]. The findings will inform decisions on modifying the definitive RCT and intervention to further improve participation and adherence. For example, the intervention duration was 6 months in this feasibility trial, and we intend to deliver a year-long intervention in the definitive RCT. The relatively short-term follow-up was another limitation in this feasibility trial, and we intend to do long-term follow-ups (at least for a year) in the definitive RCT. In terms of glycemic control, this feasibility trial gave some hints about the effectiveness of the intervention, and we intend to assess the cumulative incidence of T2DM in the definitive RCT as a co-primary outcome. We reimbursed participants’ local travel costs for attending the Yoga sessions at BNCHY, and at SVYASA, Yoga sessions were run locally and close to their residence. The reimbursement could have boosted participant recruitment and follow-up in this feasibility trial and adherence to the intervention; however, this would not be available in real-world settings.
Conclusions
The participant recruitment and follow-up and adherence to the intervention were promising in this feasibility study. In the control group, the potential contamination was low. Therefore, it should be feasible to undertake a definitive RCT in the future that will evaluate YOGA-DP’s effectiveness among high-risk individuals in India.
Change history
24 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13300-023-01420-6
References
International Diabetes Federation (IDF). IDF diabetes atlas. 9th ed. Brussels: IDF; 2019.
Anjana RM, Pradeepa R, Deepa M, et al. Prevalence of diabetes and prediabetes in urban and rural India: phase I results of the ICMR-INDIAB study. Diabetologia. 2011;54:3022–7.
National Institute for Health and Care Excellence (NICE). Preventing type 2 diabetes: risk identification and interventions for individuals at high-risk. Manchester: NICE; 2012.
Anjana RM, Shanthi Rani CS, Deepa M, et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the Chennai Urban Rural Epidemiology Study (CURES). Diabetes Care. 2015;38(8):1441–8.
Haw JS, Galaviz KI, Straus AN, et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2017;177(12):1808–17.
Galaviz KI, Weber MB, Straus A, et al. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care. 2018;41(7):1526–34.
Gong QH, Kang JF, Ying YY, et al. Lifestyle interventions for adults with impaired glucose tolerance: a systematic review and meta-analysis of the effects on glycemic control. Intern Med. 2015;54:303–10.
World Health Organization (WHO). Global strategy on diet, physical activity and health. Geneva: WHO; 2015.
Mudaliar U, Zabetian A, Goodman M, et al. Cardiometabolic risk factor changes observed in diabetes prevention programs in US settings: a systematic review and meta-analysis. PLoS Med. 2016;13(7):e1002095.
Anjana RM, Pradeepa R, Das AK, et al. Physical activity and inactivity patterns in India: results from the ICMR-INDIAB study (Phase-1) [ICMR-INDIAB-5]. Int J Behav Nutr Phys Act. 2014;11:26.
Gulati S, Misra A, Sharma M. Dietary fats and oils in India. Curr Diabetes Rev. 2017;13(5):438–43.
Minocha S, Thomas T, Kurpad AV. Are “fruits and vegetables” intake really what they seem in India? Eur J Clin Nutr. 2018;72:603–8.
Feuerstein G. The deeper dimensions of Yoga: theory and practice. Boston: Shambhala Publications; 2003.
National Health Service (NHS). A guide to Yoga: exercise. UK: NHS. 2018. https://www.nhs.uk/live-well/exercise/guide-to-yoga/. Accessed 12 Jul 2021.
Anderson JG, Taylor AG. The metabolic syndrome and mind-body therapies: a systematic review. J Nutr Metab. 2011;2011:276419.
Anjana RM, Ranjani H, Unnikrishnan R, et al. Exercise patterns and behaviour in Asian Indians: data from the baseline survey of the Diabetes Community Lifestyle Improvement Program. Diabetes Res Clin Pract. 2015;107:77–84.
Centers for Disease Control and Prevention (CDC) and American College of Sports Medicine (ACSM). General physical activities defined by level of intensity. Atlanta: CDC; 2015.
Sinha B, Ray US, Pathak A, et al. Energy cost and cardiorespiratory changes during the practice of Surya Namaskar. Indian J Physiol Pharmacol. 2004;48:184–90.
Mody BS. Acute effects of Surya Namaskar on the cardiovascular and metabolic system. J Bodyw Mov Ther. 2011;15:343–7.
Cramer H, Innes KE, Michalsen A, et al. Yoga for adults with type 2 diabetes mellitus (protocol). Cochrane Database Syst Rev. 2015;4:CD011658.
Cramer H, Lauche R, Haller H, et al. Effects of Yoga on cardiovascular disease risk factors: a systematic review and meta-analysis. Int J Cardiol. 2014;173:170–83.
Cramer H, Langhorst J, Dobos G, et al. Yoga for metabolic syndrome: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:1982–93.
Kumar V, Jagannathan A, Philip M, et al. Role of Yoga for patients with type II diabetes mellitus: a systematic review and meta-analysis. Complement Ther Med. 2016;25:104–12.
Cui J, Yan JH, Yan LM, et al. Effects of Yoga in adults with type 2 diabetes mellitus: a meta-analysis. J Diabetes Investig. 2017;8:201–9.
Chattopadhyay K, Mishra P, Manjunath NK, et al. Development of a Yoga Program for Type-2 Diabetes Prevention (YOGA-DP) among high-risk people in India. Front Public Health. 2020;8:548674.
National Institute for Health Research (NIHR). Feasibility and pilot studies. London: NIHR; 2014.
Ranjani H, Weber MB, Anjana RM, et al. Recruitment challenges in a diabetes prevention trial in a low- and middle-income setting. Diabetes Res Clin Pract. 2015;110:51–9.
Chattopadhyay K, Mishra P, Singh K, et al. Yoga Programme for Type-2 Diabetes Prevention (YOGA-DP) among high risk people in India: a multicentre feasibility randomised controlled trial protocol. BMJ Open. 2020;10(9):e036277.
Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1):1.
Hooper R. Justifying sample size for a feasibility study. London: NIHR; 2014.
Horovskaia LA, Lobachevskaia TV, Chernichuk OV. The analytical quality and comparability value of four glucometers from different manufacturers. Klin Lab Diagn. 2015;60(1):60–3.
Baumstark A, Jendrike N, Kamecke U, et al. Measurement accuracy of two professional-use systems for point-of-care testing of blood glucose. Clin Chem Lab Med. 2020;58(3):445–55.
Buysschaert M, Medina JL, Buysschaert B, et al. Definitions (and current controversies) of diabetes and prediabetes. Curr Diabetes Rev. 2016;12:8–13.
Adams R. Revised Physical Activity Readiness Questionnaire. Can Fam Physician. 1999;45:992, 995, 1004–5.
Craig CL, Marshall AL, Sjöström M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36.
Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44:227–39.
Bandura A. Guide for constructing self-efficacy scales. In: Pajares F, Urdan T, editors. Self-efficacy beliefs of adolescents. Greenwich: Information Age Publishing; 2006. p. 307–37.
Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239.
Douglas A, Bhopal RS, Bhopal R, et al. Recruiting South Asians to a lifestyle intervention trial: experiences and lessons from PODOSA (Prevention of Diabetes & Obesity in South Asians). Trials. 2011;12:220.
Cramer H, Haller H, Dobos G, et al. A systematic review and meta-analysis estimating the expected dropout rates in randomized controlled trials on Yoga interventions. Evid Based Complement Alternat Med. 2016;2016:5859729.
Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187–94.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Ramachandran A, Snehalatha C, Mary S, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–97.
Ramachandran A, Snehalatha C, Ram J, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013;1(3):191–8.
Glechner A, Keuchel L, Affengruber L, et al. Effects of lifestyle changes on adults with prediabetes: a systematic review and meta-analysis. Prim Care Diabetes. 2018;12(5):393–408.
Uusitupa M, Khan TA, Viguiliouk E, et al. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis. Nutrients. 2019;11(11):2611.
European Medicines Agency (EMA). Guideline on clinical evaluation of medicinal products used in weight management. London: EMA; 2016.
Birdee GS, Legedza AT, Saper RB, et al. Characteristics of Yoga users: results of a national survey. J Gen Intern Med. 2008;23:1653–8.
Ding D, Stamatakis E. Yoga practice in England 1997–2008: prevalence, temporal trends, and correlates of participation. BMC Res Notes. 2014;7:172.
Mishra P, Greenfield SM, Harris T, et al. Yoga program for type 2 diabetes prevention (YOGA-DP) among high-risk people: qualitative study to explore reasons for non-participation in a feasibility randomized controlled trial in India. Front Public Health. 2021;9:682203.
Mishra P, Harris T, Greenfield SM, et al. Feasibility trial of Yoga programme for type 2 diabetes prevention (YOGA-DP) among high-risk people in India: a qualitative study to explore participants’ trial- and intervention-related barriers and facilitators. Int J Environ Res Public Health. 2022;19(9):5514.
Acknowledgment
The authors would like to thank the funding agencies, participants, and TSC members, namely Prof. Nitin Kapoor (chair), Prof. Mohammed K Ali, Dr. Ajay Vamadevan, and Dr. Dimple Kondal.
Funding
The study was funded by the UK’s FCDO/MRC/NIHR/Wellcome Trust Joint Global Health Trials (MR/R018278/1). The funding agencies had no role in designing the study or in writing the manuscript. The publication fee was covered through the UKRI block grant.
Author Contributions
KC wrote the first draft of the manuscript. PM, KavS, KalS, TH, MH, SMG, NKM, RN, SM, NT, SAL, SK, and DP contributed significantly to the revision of the manuscript. All the authors read and approved the final manuscript.
List of Investigators
KC (Principal Investigator) conceptualized and designed the study with the help of Co-Investigators (TH, MH, SMG, NKM, NT, SAL, SK, and DP).
Prior Presentation
The study abstract was selected for presentation at the International Diabetes Federation (IDF) Congress 2021 (held virtually from 6 to 11 December 2021 due to the COVID-19 pandemic) and published in the event material. https://doi.org/10.26226/morressier.617c37317c09fc044a9751b2.
Disclosures
KC, PM, KavS, KalS, TH, MH, SMG, NKM, RN, SM, NT, SAL, SK, and DP have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki. The research ethics committees of the following institutes gave ethics approval: Faculty of Medicine and Health Sciences, University of Nottingham, UK (14-1805); CCDC, India (CCDC_IEC_09_2018); BNCHY, India (BNCHY/IEC/2/2019); and S-VYASA, India (RES/IEC-SVYASA/138/2018). We obtained written informed consent from participants.
Data Availability
The data set generated during the current study is available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The original online version of this article was revised due to update in author group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chattopadhyay, K., Mishra, P., Singh, K. et al. Yoga Programme for Type 2 Diabetes Prevention (YOGA-DP) Among High-Risk People in India: A Multicenter Feasibility Randomized Controlled Trial. Diabetes Ther 14, 1137–1154 (2023). https://doi.org/10.1007/s13300-023-01395-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01395-4