Abstract
Introduction
Albuminuria, or elevated urinary albumin-to-creatine ratio (UACR), is a biomarker for chronic kidney disease that is routinely monitored in patients with type 2 diabetes (T2D). Head-to-head comparisons of novel antidiabetic drugs on albuminuria outcomes remain limited. This systematic review qualitatively compared the efficacy of novel antidiabetic drugs on improving albuminuria outcomes in patients with T2D.
Methods
We searched the MEDLINE database until December 2022 for Phase 3 or 4 randomized, placebo-controlled trials that evaluated the effects of sodium-glucose co-transporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and dipeptidyl peptidase-4 (DPP-4) inhibitors on changes in UACR and albuminuria categories in patients with T2D.
Results
Among 211 records identified, 27 were included, which reported on 16 trials. SGLT2 inhibitors and GLP-1 RAs decreased UACR by 19–22% and 17–33%, respectively, versus placebo (P < 0.05 for all studies) over median follow-up of ≥ 2 years; DPP-4 inhibitors showed varying effects on UACR. Compared with placebo, SGLT2 inhibitors decreased the risk for albuminuria onset by 16–20% and for albuminuria progression by 27–48% (P < 0.05 for all studies) and promoted albuminuria regression (P < 0.05 for all studies) over median follow-up of ≥ 2 years. Evidence on changes in albuminuria categories with GLP-1 RA or DPP-4 inhibitor treatment were limited with varying outcome definitions across studies and potential drug-specific effects within each class. The effect of novel antidiabetic drugs on UACR or albuminuria outcomes at ≤ 1 year remains poorly studied.
Conclusion
Among the novel antidiabetic drugs, SGLT2 inhibitors consistently improved UACR and albuminuria outcomes in patients with T2D, with continuous treatment showing long-term benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Novel antidiabetic drugs showed renoprotective effects in patients with type 2 diabetes (T2D) based on hard renal composite endpoints in clinical trials |
In terms of risk reduction of the hard renal composite endpoint, sodium-glucose co-transporter-2 (SGLT2) inhibitors showed the greatest benefit versus placebo in patients with T2D, followed by glucagon-like peptide-1 receptor agonists (GLP-1 RAs), while dipeptidyl peptidase-4 (DPP-4) inhibitors did not show a benefit |
Unlike for hard renal endpoints, head-to-head comparisons of the efficacies of novel antidiabetic drugs on urinary albumin-to-creatine ratio (UACR) or albuminuria outcomes remain limited despite the recommended routine monitoring of these renal disease risk markers in T2D clinical practice |
The qualitative synthesis of available evidence revealed that SGLT2 inhibitors consistently improved UACR and albuminuria outcomes in patients with T2D, while evidence on the benefit of GLP-1 RAs and DPP-4 inhibitors was relatively limited and showed potential drug-specific effects within the classes |
Continuous management of albuminuria with specific novel antidiabetic drugs, particularly SGLT2 inhibitors, together with routine UACR monitoring, could delay the onset and progression of renal disease in patients with T2D |
Introduction
Globally, approximately 537 million adults lived with diabetes in 2021, with most having type 2 diabetes (T2D) [1]. Chronic kidney disease (CKD) is a major complication of T2D, with a reported prevalence of 24–50% in this population [2,3,4,5]. CKD manifests clinically as persistent (> 3 months) albuminuria and/or impaired estimated glomerular filtration rate (eGFR) that eventually progresses to end-stage kidney disease (ESKD) [6] and is associated with increased risk of cardiovascular (CV) and all-cause mortality in patients with T2D [7, 8].
The rate of CKD progression in patients with T2D is highly variable, being characterized by different rate trajectories of renal function decline measured by the eGFR [9]. Albuminuria, indicated by elevated urinary albumin-to-creatine ratio (UACR), is a major risk factor for rapid renal function decline and progression to ESKD [10,11,12,13,14,15,16,17,18,19,20,21]. Albuminuria or increased UACR is also an important predictor of adverse outcomes, including CV events and mortality [10, 11, 16, 21]. The routine screening of UACR, together with eGFR, from the time of T2D diagnosis is recommended by clinical guidelines to detect CKD and monitor its progression [6, 22, 23].
The novel antidiabetic drugs, sodium-glucose co-transporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1 RAs), have been shown to confer CV- and renoprotection in patients with T2D in CV and renal outcomes-focused trials and are recommended by clinical guidelines for the treatment of patients with T2D and risks for CV disease and/or CKD [23,24,25,26]. Dipeptidyl peptidase-4 (DPP-4) inhibitors showed a neutral effect in reducing the risks of renal and CV events [27, 28]. A recent network meta-analysis of 16 randomized, placebo-controlled trials showed that treatment with SGLT2 inhibitors resulted in the greatest risk reduction for CV and renal events, including the hard renal composite endpoint (doubling of serum creatinine, ≥ 40% decline in eGFR, development of ESKD and death due to renal causes), followed by GLP-1 RAs, while DPP-4 inhibitors did not improve these outcomes compared with placebo [29].
Although hard renal endpoints were frequently used in clinical trials to assess the renoprotective effects of novel antidiabetic drugs, limitations for their use include the need for a large study cohort and a long follow-up period [30]. UACR or albuminuria, being routinely determined in clinical practice, as well as an important predictor of renal function decline and clinical outcomes, is increasingly recognized as a potential short-term surrogate renal endpoint for the evaluation of novel antidiabetic drugs in T2D clinical trials [30,31,32]. To our knowledge, head-to-head comparison studies on the efficacy of novel antidiabetic drugs in improving UACR and albuminuria outcomes remain limited [33, 34]. We aimed to compare the efficacy of novel antidiabetic drug classes, namely SGLT2 inhibitors, GLP-1 RAs and DPP-4 inhibitors, on UACR and albuminuria outcomes in patients with T2D based on available clinical data.
Methods
Search Strategy
We conducted a systematic search of the MEDLINE database (via PubMed) to identify relevant randomized controlled trials (RCTs) published from inception to 5 December 2022. Only English-language articles were retrieved; we did not limit the search by text availability, article type or any other trial characteristics. The following search terms were used: (“sodium-glucose transporter 2 inhibitors” [Mesh] OR “dipeptidyl-peptidase IV inhibitors” [Mesh] OR (“glucagon-like peptide 1” [Mesh] OR “glucagon-like peptide-1 receptor” [Mesh])) AND (“diabetes mellitus, type 2” [Mesh]) AND (UACR OR (“Albuminuria” [Mesh]) AND ((randomized controlled) OR (randomized controlled trial)). To ensure completeness of the search, different entry terms for each aforementioned phrase or word, as well as the individual drugs in each class were included in the search string (Supplementary Table S1). We also manually screened the references of the systematic reviews, meta-analyses and pooled analyses shortlisted from the database search to identify additional relevant RCTs that were not initially captured by the search string.
Study Selection
The retrieved studies were included if they met the following criteria, based on the patient, intervention, comparison, and outcome (PICO) framework: (1) Phase 3 or 4 RCT; (2) enrolled adult patients with T2D regardless of concomitant CV disease, renal impairment and/or albuminuria; (3) evaluated SGLT2 inhibitors, GLP-1 RAs or DPP-4 inhibitors versus placebo; (4) reported UACR and/or albuminuria outcomes.
The articles retrieved from the database search were initially screened for eligibility based on the study type, patient population, intervention and comparator in their titles and abstracts. The shortlisted articles then underwent full text screening for all eligibility criteria specified, including study outcomes. For RCTs determined to be eligible, we additionally sourced for their primary, secondary, post hoc and/or exploratory analyses that were not initially captured by the database search and assessed the eligibility of each article for inclusion.
Data Extraction
The data extracted for each eligible study included patient characteristics, dosage of the intervention, study duration or median follow-up period (categorized as short term [≤ 1 year] and long term [≥ 2 years]) and outcomes of interest. Data extraction was conducted using a standardized Excel spreadsheet by one researcher and independently reviewed by two others.
Outcomes
Outcomes of interest were (1) changes in UACR from baseline over time (continuous) and (2) changes in albuminuria stages (categorical), namely normoalbuminuria (UACR < 30 mg/g), microalbuminuria (UACR 30–300 mg/g) and macroalbuminuria (UACR > 300 mg/g) [6, 35]. Albuminuria onset was defined as the shift to micro- or macroalbuminuria in patients with normoalbuminuria at baseline; albuminuria progression as the shift to macroalbuminuria in patients with microalbuminuria at baseline; and albuminuria regression as the shift to normo- or microalbuminuria in patients with macroalbuminuria at baseline or the shift to normoalbuminuria in those with microalbuminuria at baseline.
Due to heterogeneity of the trial characteristics (e.g., study duration) and the outcome definitions or measures, a meta-analysis was not considered meaningful. Instead, albuminuria outcomes were qualitatively synthesized based on study duration. Analyses of albuminuria outcomes in subgroups defined by baseline eGFR or albuminuria status were also extracted from the selected studies and summarized.
Risk of Bias Assessment
The risk of bias for included studies was assessed by two independent reviewers, separately for continuous and categorical albuminuria outcomes using the Cochrane Risk of Bias tool.
Compliance with Ethics Guidelines
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [36]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study Selection
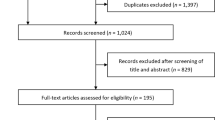
Among 211 records identified from the database search, 75 were shortlisted from title and abstract screening (Supplementary Fig. S1). An additional 25 records not identified from the database search were included for completeness. Hence, a total of 100 records underwent full text screening, from which 53 were deemed eligible. Twelve of the selected records were further excluded during data extraction. Finally, data were extracted from 41 articles that reported on 26 RCTs.
Among the 26 selected RCTs, 16 had enrolled patients with T2D regardless of baseline renal impairment and/or albuminuria status, while 10 enrolled patients with concomitant CKD. To ensure the homogeneity of the study population reported in this systematic review, herein, only the results in patients with T2D regardless of baseline CKD status (27 articles from 16 RCTs) were qualitatively synthesized. The risk of bias assessment of the included studies is presented in Supplementary Fig. S2.
Study Characteristics
The characteristics of the studies included in this qualitative synthesis are summarized in Table 1. Patients had concomitant CV disease or CV risk factors or were overweight/obese. Eleven studies involved long-term follow-up (median ≥ 2 years): three on SGLT2 inhibitors (CANVAS PROGRAM [37,38,39,40,41], DECLARE-TIMI-58 [42] and EMPA-REG-OUTCOME [43,44,45,46]), five on GLP-1 RAs (AMPLITUDE-O [47], ELIXA [48, 49], LEADER [50, 51], REWIND [52] and SUSTAIN-6 [53, 54]), and three on DPP-4 inhibitors (CARMELINA [55,56,57], SAVOR-TIMI-53 [58] and TECOS [59]), while five studies involved short-term follow-up (≤ 1 year): two on SGLT2 inhibitors (DECREASE [60] and EMPEROR-Pooled [61]), four on GLP-1 RAs (DECREASE [60], EXSCEL [62], SAFEGUARD [63] and SCALE [64]), and one on a DPP-4 inhibitor (SAFEGUARD [63]); the DECREASE and SAFEGUARD studies evaluated two different drug classes each in separate arms [60, 63]. Nine studies, all of which were of long-term duration, investigated albuminuria outcomes in subgroups defined by baseline albuminuria and/or eGFR status (Table 1).
Effects of Novel Antidiabetic Drugs on Changes in UACR
Overall T2D Population
The effects of novel antidiabetic drugs on changes in UACR were mainly reported in studies with long-term follow-up (Table 2). SGLT2 inhibitors (canagliflozin and empagliflozin) decreased UACR by 19–22% relative to placebo (P < 0.05 for all studies) [37, 40, 44]. GLP-1 RAs decreased UACR by 17–33% (P < 0.05 for all studies) [47, 50, 52, 54]. Lixisenatide also showed a significant benefit in slowing the increase in UACR versus placebo (P = 0.004) [49]. The effect of different DPP-4 inhibitors on UACR varied greatly. Saxagliptin showed the greatest magnitude of UACR reduction (− 34.3 mg/g; P < 0.004) relative to placebo, followed by sitagliptin at − 0.18 mg/g (P = 0.031) [58, 59]. Conversely, no meaningful difference in UACR (− 0.05 mg/g) was seen with linagliptin versus placebo [55].
Data on the short-term effects of novel antidiabetic drugs on UACR outcomes were limited. At 52 weeks, empagliflozin decreased UACR by 19% compared with placebo [61]. At 56 weeks, liraglutide (3.0 mg once daily [qd]) decreased UACR by 20% versus placebo, although a statistically significant difference was not observed at the 1.8 mg qd dose [64]. Nevertheless, in a post hoc analysis of the LEADER study at 1 year, UACR was decreased by 15% (95% confidence interval [CI] 13, 18) in patients treated with liraglutide (0.6–1.8 mg qd) and increased by 10% (95% CI 7, 14) in those receiving placebo [51]. At a shorter follow-up duration of 16 weeks, the DECREASE study showed that dapagliflozin and exenatide resulted in higher, but statistically nonsignificant, percentage decreases in UACR compared with placebo [60]. In the 12-week SAFEGUARD study, UACR decreased by 24% and 32% with liraglutide and sitagliptin, respectively, versus placebo, although the differences were not statistically significant [63].
Subgroup Analysis by Baseline Albuminuria Status
Two SGLT2 inhibitor studies (CANVAS PROGRAM and EMPA-REG OUTCOME) and two GLP-1 RA studies (ELIXA and LEADER) reported UACR changes by baseline albuminuria status during long-term follow-up; no relevant DPP-4 inhibitor study was identified (Supplementary Table S2). SGLT2 inhibitors lowered UACR compared with placebo regardless of baseline albuminuria status (P < 0.05 for all albuminuria subgroups in both CANVAS PROGRAM and EMPA-REG OUTCOME), although the magnitude of the treatment effect was observed to be greater in patients with microalbuminuria (− 34 to − 30%) or macroalbuminuria (− 36 to − 32%) versus those without (− 12 to − 9%) [40, 43]. Conversely, GLP-1 RAs showed varying effects on UACR reduction by baseline albuminuria status. Liraglutide decreased UACR relative to placebo by 14%, 24% and 13% in patients with normo-, micro- and macroalbuminuria, respectively, although the between-group difference only reached statistical significance in patients with normo- and microalbuminuria [50]. Compared with placebo, lixisenatide significantly decreased UACR by 39.18% (P = 0.0070) in the macroalbuminuria subgroup; the reduction in UACR was smaller and nonsignificant in the microalbuminuria subgroup (− 21.10%; P = 0.0502) and marginal in the normoalbuminuria subgroup (− 1.69%; P = 0.7398) [48].
Subgroup Analysis by Baseline eGFR Status
Five studies investigated the effects of the three novel antidiabetic drug classes on changes in UACR according to baseline eGFR (< 60 and ≥ 60 ml/min/1.73 m2) over long-term follow-up (Supplementary Table S3). The SGLT2 inhibitor, canagliflozin, led to UACR reduction in both baseline eGFR subgroups. UACR was reduced by 13 to 26% in patients with eGFR > 30 to < 60 ml/min/1.73 m2 and by 17% in those with eGFR ≥ 60 ml/min/1.73 m2 [41]. The effect of a GLP-1 RA, liraglutide, on UACR changes differed by baseline eGFR status as reported in the LEADER study. Liraglutide decreased UACR by 10–24% versus placebo across all eGFR subgroups, although statistical significance was only observed among patients with eGFR ≥ 60 ml/min/1.73 m2 [50]. Subgroup analyses by baseline eGFR status revealed varying results with DPP-4 inhibitors. The magnitudes of mean UACR change from baseline with saxagliptin were greater in the eGFR < 30 and 30–50 ml/min/1.73 m2 subgroups at − 245.2 mg/g and − 105.0 mg/g, respectively, although in the former subgroup it did not reach statistical significance; a smaller but significant improvement in UACR (− 19.3 mg/g; P = 0.033) was observed in the eGFR > 50 ml/min/1.73 m2 subgroup [58]. In contrast, linagliptin and sitagliptin did not lead to statistically significant improvements in UACR versus placebo across any of the eGFR subgroups [55, 59]. The effect on UACR changes in the eGFR < 30 ml/min/1.73 m2 subgroup was reported only for GLP-1 RAs and DPP-4 inhibitors, although no statistically significant benefit was observed [50, 55, 58].
Effects of Novel Antidiabetic Drugs on Changes in Albuminuria Categories
Overall T2D Population (by Baseline Albuminuria Status)
Among the selected T2D studies that evaluated the effect of novel antidiabetic drugs on changes in albuminuria status, all except one involved long-term follow-up (Tables 3, 4, 5). Albuminuria onset was reported for SGLT2 inhibitors and GLP-1 RAs, but not DPP-4 inhibitors (Table 3). Compared with placebo, SGLT2 inhibitors significantly decreased the risk for albuminuria onset by 16–20%, that for microalbuminuria onset by 20–21% and that for macroalbuminuria onset by 21–42% (P < 0.05 for all studies) [40, 42, 43]. GLP-1 RA studies mainly reported effects on macroalbuminuria onset. Among GLP-1 RAs, dulaglutide, liraglutide and semaglutide significantly decreased the risk for macroalbuminuria onset by 22–46% compared with placebo (P < 0.05 for all studies) [50, 52, 53]. The decrease in risk for macroalbuminuria onset with lixisenatide was, however, not statistically significant [48]. EXSCEL was the only short-term study identified where exenatide significantly reduced the proportion of patients with albuminuria onset versus placebo (4.0% versus 3.0%; P = 0.03) at 6 months; this was driven by a decrease in the incidence of microalbuminuria onset (2.9% versus 2.0%; P = 0.02) rather than macroalbuminuria onset (1.1% versus 1.0%; P = 0.79) [62].
Albuminuria progression was defined differently across studies, for example, some studies considered deterioration in albuminuria categories among patients with normo- or microalbuminuria, i.e., combined albuminuria onset and progression (Table 4). Among patients with baseline microalbuminuria, SGLT2 inhibitors significantly lowered the risk for albuminuria progression compared with placebo [38, 42, 46]. The greatest risk reduction in albuminuria progression of approximately 50% (P < 0.0001) was observed with dapagliflozin, followed by empagliflozin and canagliflozin, which brought about risk reductions of 38% (P < 0.001) and 27% (P < 0.05), respectively [38, 42, 46]. Furthermore, the risk of combined albuminuria onset (in patients with baseline normoalbuminuria) and progression (in patients with baseline microalbuminuria) was significantly reduced with SGLT2 inhibitors (dapagliflozin: hazard ratio [HR] 0.82 [95% CI 0.77, 0.88]; empagliflozin: odds ratio 0.67 [95% CI 0.59, 0.77]) versus placebo [42, 43]. The risk of progression to macroalbuminuria in patients with baseline normoalbuminuria and microalbuminuria (i.e., combined macroalbuminuria onset and albuminuria progression) was also significantly decreased by 55% (95% CI 44, 63) with empagliflozin versus placebo [43, 45]. The GLP-1 RA, dulaglutide, significantly reduced the risk for albuminuria progression by 24% (95% CI 11, 35) compared with placebo [52]. While efpeglenatide led to lower rates of combined macroalbuminuria onset and albuminuria progression (32% risk reduction) [47], lixisenatide did not result in a statistically significant difference on this outcome (P = 0.0908) [48]. A DPP-4 inhibitor study revealed that linagliptin significantly reduced the risk of combined albuminuria onset and progression by 14% (P = 0.003) compared with placebo [56].
The effect of novel antidiabetic drugs on albuminuria regression, including microalbuminuria and macroalbuminuria regression, is shown in Table 5. Compared with placebo, SGLT2 inhibitors were significantly associated with albuminuria regression (HR 1.70 [95% CI 1.51, 1.91] for albuminuria regression with canagliflozin; HR 1.46 [95% CI 1.31, 1.62] and 1.82 [95% CI 1.51, 2.20] for microalbuminuria and macroalbuminuria regression with dapagliflozin, respectively; HR 1.43 [95% CI 1.22, 1.67] and 1.82 [95% CI 1.40, 2.37] for microalbuminuria and macroalbuminuria regression with empagliflozin, respectively) [38, 42, 43]. In contrast, available studies did not show statistically significant macroalbuminuria regression with the GLP-1 RA, lixisenatide (P = 0.4086), and the DPP-4 inhibitor, linagliptin (P = 0.31), compared with placebo [48, 57].
Subgroup Analysis by Baseline eGFR Status
Subgroup analyses of changes in albuminuria categories by baseline eGFR status were available for a GLP-1 RA and a DPP-4 inhibitor in two studies with long-term follow-up (Supplementary Table S4). Compared with placebo, the GLP-1 RA, dulaglutide, significantly decreased the risk of combined albuminuria onset and progression (HR 0.70 [95% CI 0.59, 0.81]) in the eGFR ≥ 60 ml/min/1.73 m2 group, while a smaller nonsignificant decrease in risk (HR 0.91 [95% CI 0.73, 1.13]) was observed among patients with eGFR < 60 ml/min/1.73 m2 [52]. Linagliptin did not show a statistically significant association with albuminuria regression versus placebo across all eGFR subgroups [55].
Discussion
To our knowledge, this is, to date, the most comprehensive qualitative evidence synthesis of albuminuria outcomes (both continuous and categorical) in patients with T2D treated with novel antidiabetic drugs. A previous meta-analysis of 26 studies (N = 14,929) compared the effect of novel antidiabetic drugs on percentage changes in albuminuria as a continuous outcome (measured by either urinary albumin excretion or UACR) versus placebo or other conventional glucose-lowering drugs [34]. Another meta-analysis of 19 studies (N = 140,851) compared the effect of the novel antidiabetic drugs on albuminuria progression and regression as categorical outcomes [33]. Results from the present qualitative synthesis and prior meta-analyses provide evidence on the comparative efficacies of novel antidiabetic drugs in improving UACR and albuminuria outcomes, beyond the risk reduction for hard renal endpoints, which will further inform the use of these interventions for the prevention and management of CKD in patients with T2D.
Effects of Novel Antidiabetic Drugs on Changes in UACR
Changes in UACR over time with novel antidiabetic drug treatment were reported using different outcome measures (as absolute mean, absolute geometric mean and percentage) across the selected studies, thus precluding a meaningful meta-analysis. SGLT2 inhibitors and GLP-1 RAs showed benefit in improving UACR outcomes in patients with T2D during long-term follow-up, while varying effects were observed for DPP-4 inhibitors [37, 40, 44, 47, 49, 50, 52, 54, 55, 58, 59]. The short-term effects of novel antidiabetic drugs on changes in UACR have not been adequately studied, being reported in only five trials [51, 60, 61, 63, 64]. At around 1 year, empagliflozin and liraglutide showed benefit in reducing UACR versus placebo [51, 61, 64]. The nonsignificant benefit of novel antidiabetic drugs in improving UACR outcomes at 12–16 weeks of the DECREASE and SAFEGUARD trials may be due to their relatively small sample sizes [60, 63]. Hence, the benefit of novel antidiabetic drugs in improving UACR outcomes in the shorter term (≤ 1 year) warrants further investigation.
When analyzed by baseline albuminuria categories, SGLT2 inhibitors were the only class of novel antidiabetic drugs that led to significantly decreased UACR versus placebo across all subgroups [39, 40, 43]. The treatment effect of SGLT2 inhibitors on UACR was observed to be greater in patients with albuminuria than in those without [39, 40, 43]. These findings were corroborated by studies in patients with T2D and concomitant albuminuria (CREDENCE, DAPA-CKD and Study MB102029), where UACR was consistently shown to be decreased with SGLT2 inhibitors versus placebo [65,66,67]. In contrast, GLP-1 RAs showed varying effects on UACR in different baseline albuminuria subgroups, although this may be due to the small subgroup sample sizes and warrants further investigation [48, 50]. No subgroup analysis of UACR changes with DPP-4 inhibitors by baseline albuminuria status has been identified to date.
When analyzed by baseline eGFR, canagliflozin showed statistically significant UACR improvement across all subgroups [41]. Consistently, findings from CREDENCE, DAPA-CKD and Study MB102029 showed that SGLT2 inhibitors improved UACR versus placebo among enrolled patients with T2D and concomitant renal impairment (eGFR > 25 to < 90 ml/min/1.73 m2) [65, 67,68,69]. Furthermore, similar to the findings in CANVAS PROGRAM, a greater magnitude of UACR reduction was observed in the subgroup with eGFR < 60 versus ≥ 60 ml/min/1.73 m2 in CREDENCE [41, 68]. Despite showing a benefit in the overall study cohort, the GLP-1 RA, liraglutide, and the DPP-4 inhibitor, saxagliptin, did not lead to statistically significant UACR reductions in subgroups with eGFR < 60 and < 30 ml/min/1.73 m2, respectively [50, 58]. Small subgroup sample sizes, which may not be sufficiently powered to detect treatment effects on UACR cannot be ruled out and necessitates further investigation. Similarly, the subtle treatment benefit observed with sitagliptin in the overall study cohort lost statistical significance in the subgroup analysis, likely because of the reduced sample sizes [59].
Our observations on the comparative efficacy of novel antidiabetic drugs on UACR changes in the overall T2D population corroborated the conclusions of an earlier meta-analysis by Luo et al. [34]. They determined that both SGLT2 inhibitors and GLP-1 RAs significantly reduced UACR compared with other conventional therapies or placebo and that SGLT2 inhibitors showed a greater magnitude of UACR reduction than GLP-1 RAs (weighted mean difference [WMD] − 26.23% versus − 13.85%) [34]. While all SGLT2 inhibitors studied led to UACR reduction, a statistically significant effect was not observed with lixisenatide among GLP-1 RAs [34]. UACR reduction with DPP-4 inhibitors (WMD − 6.19%) compared with other conventional therapies or placebo was not statistically significant [34]. Furthermore, subgroup analysis from Luo et al. revealed that SGLT2 inhibitors led to UACR reduction regardless of baseline albuminuria status, with a greater effect in patients with albuminuria versus those without, consistent with the observations from the present evidence synthesis [34]. GLP-1 RAs also decreased UACR across all baseline albuminuria subgroups with the greatest benefit being observed among patients with macroalbuminuria [34]. SGLT2 inhibitors decreased UACR in patients with eGFR ≥ 60 ml/min/1.73 m2, with a greater reduction in patients with eGFR > 60 to 90 ml/min/1.73 m2 than in those with eGFR > 90 ml/min/1.73 m2 (− 33.36% versus − 9.40%, P < 0.05 for both). However, no statistically significant treatment benefit was seen in patients with eGFR 30–60 ml/min/1.73 m2, although a wide 95% CI was noted (–35.59% [95% CI –83.11, 11.94]) [34]. GLP-1 RAs significantly decreased UACR only in patients with eGFR > 60 to 90 ml/min/1.73 m2, with numerical improvements observed for those with eGFR 30–60 and > 90 ml/min/1.73 m2 [34]. Notably, although UACR outcomes with novel antidiabetic drug treatment reported in Luo et al. were generally consistent with those observed in the present systematic review, the meta-analysis by Luo et al. evaluated a different set of trials [34]. First, recent CV and renal outcomes-focused trials were included in the present systematic review while several glycemic control trials were included in the prior meta-analysis by Luo et al. Second, the present systematic review focused on studies in the overall T2D population and excluded those that enrolled only patients with T2D and concomitant CKD for a more generalizable and uniform study cohort, unlike in the meta-analysis by Luo et al. Last, in addition to placebo-controlled RCTs, those evaluating novel antidiabetic drugs against conventional therapies were included in the meta-analysis by Luo et al.
Effects of Novel Antidiabetic Drugs on Changes in Albuminuria Categories
Albuminuria onset, progression and regression were defined differently across available studies; standardized definitions of these outcomes in future studies will allow comparisons among novel antidiabetic drug classes via a meta-analysis. Changes in albuminuria categories with novel antidiabetic drugs were mainly reported in studies with long-term follow-up. EXSCEL was the only study shortlisted from the systematic search that had a time point of < 1 year, showing reduced microalbuminuria onset risk with exenatide versus placebo; effects on albuminuria progression and regression over short-term follow-up were not reported [62].
Compared with placebo, SGLT2 inhibitors showed significant benefit in reducing the risks for albuminuria onset as well as albuminuria progression [38, 40, 42, 43, 45, 46]. In contrast to SGLT2 inhibitor studies, GLP-1 RA and DPP4 inhibitor studies used varying outcome definitions besides those defined in this systematic review, which included microalbuminuria onset, macroalbuminuria onset, deterioration in albuminuria categories among patients with normo- and microalbuminuria (i.e., combined albuminuria onset and progression) and incident macroalbuminuria in patients with normo- and microalbuminuria (i.e., combined macroalbuminuria onset and albuminuria progression). Among GLP-1 RAs, lixisenatide failed to show a statistically significant benefit in reducing the risk for deterioration in UACR categories [48], which may indicate a drug-specific effect within the class. Liraglutide, dulaglutide and semaglutide were shown to reduce the risks for micro- or macroalbuminuria onset [50, 52, 53]. The risk for albuminuria progression was reduced with dulaglutide [52] and that for combined macroalbuminuria onset and albuminuria progression was reduced with efpeglenatide [47]. Available data for DPP-4 inhibitors remained limited with only one relevant study (CARMELINA) identified that showed the reduced risk for combined albuminuria onset and progression with linagliptin [56].
SGLT2 inhibitors were associated with albuminuria regression (including that for micro- and macroalbuminuria) [38, 42, 43, 45]. Consistently, the DAPA-CKD study, which recruited patients with T2D and albuminuria (UACR between 200 and 5000 mg/g), showed that dapagliflozin was associated with reduced albuminuria progression (HR 0.39 [95% CI 0.29, 0.51]) and increased macroalbuminuria regression (HR 2.06 [95% CI 1.78, 2.39]) [67]. In contrast, the limited evidence available did not reveal a significant benefit of a GLP-1 RA (lixisenatide) and a DPP-4 inhibitor (linagliptin) in promoting macroalbuminuria regression; there were no reports on the effect of these two drug classes on albuminuria regression or microalbuminuria regression [48, 57].
Reports on the effect of novel antidiabetic drugs on categorical changes in albuminuria by baseline eGFR status were scarce. The reduction in the risk for combined albuminuria onset and progression with dulaglutide was statistically significant only in patients with eGFR ≥ 60 ml/min/1.73 m2, which was consistent with findings from the eGFR subgroup analysis of UACR changes with another GLP-1 RA, liraglutide [50, 52]. A DPP-4 inhibitor, linagliptin, was not associated with albuminuria regression across all baseline eGFR subgroups, which corroborated its lack of effect on UACR and albuminuria changes in the overall study cohort [55, 57]. Although no eGFR subgroup analyses of albuminuria regression with SGLT2 inhibitors were identified, the DAPA-CKD trial showed that dapagliflozin induced macroalbuminuria regression (HR 2.06 [95% CI 1.78, 2.39]) in enrolled patients with T2D and impaired renal function (eGFR >25 to <75 mL/min/1.73m2) [67].
The efficacy of novel antidiabetic drugs on albuminuria progression and regression was also compared in a meta-analysis by Chewcharat et al., where all three novel antidiabetic drug classes significantly decreased the risk for albuminuria progression compared with placebo (by 30% for SGLT2 inhibitors, 24% for GLP-1 RAs and 11% for DPP-4 inhibitors) [33]. Furthermore, higher HRs for albuminuria regression were observed with SGLT2 inhibitors (HR 1.73 [95% CI 1.54, 1.94]; P < 0.001) and DPP-4 inhibitors (HR 1.20 [95% CI 1.02, 1.40; P = 0.027) compared with placebo [33]. Comparatively, the present qualitative review revealed limited evidence and benefit of GLP-1 RAs and DPP-4 inhibitors in preventing albuminuria progression or promoting albuminuria regression. This difference can be attributed to the inclusion of a different set of studies; the present review compared studies with standard defined outcomes in the overall T2D population over long-term follow-up durations (≥ 2 years). Furthermore, a meta-analysis captures the statistical combination of results across the different studies, which may not reflect outcomes of the individual studies as reported in the present qualitative review. GLP-1 RA studies analyzed in Chewcharat et al. that were excluded for the evaluation of albuminuria progression in the present review were SAFEGUARD, LEADER and EXSCEL; DPP-4 inhibitor studies that were excluded were TECOS, SAVOR-TIMI-53, SAFEGUARD and Cooper et al. [33]. Reasons for the exclusion of these studies were differences in study type, population, follow-up duration and outcome definition [50, 51, 58, 59, 62, 63, 70, 71]. The effect of SGLT2 inhibitors in inducing albuminuria regression was based on the CANVAS PROGRAM and Study MB102029 in the meta-analysis by Chewcharat et al. [33]. We identified additional studies, namely DECLARE-TIMI-58 and EMPA-REG-OUTCOME, which supported the same conclusion [42, 43, 45]. The benefit of DPP-4 inhibitors in promoting albuminuria regression, as reported by Chewcharat et al., was based on MARLINA-T2D and SAVOR-TIMI-53 [33]. However, albuminuria regression results from these DPP-4 inhibitor studies were not extracted for comparison in this review because MARLINA-T2D specifically enrolled patients with T2D and concomitant CKD and had a short-term (24 weeks) follow-up duration, and SAVOR-TIMI-53 only presented the number of patients in each UACR category at baseline and end of treatment, without the HRs and 95% CIs for changes in UACR categories [58, 72].
Limitations
A limitation lies in the heterogeneous definitions and measures of the UACR and albuminuria outcomes in the selected RCTs, which did not allow for a meaningful meta-analysis. The differences in the magnitudes of treatment effect among the novel antidiabetic drug classes cannot be quantitatively compared. Furthermore, changes in UACR and albuminuria were post hoc and/or exploratory outcomes in some of the included studies. Post hoc outcomes may be biased because of the possibility of data selection after unblinding, as indicated in the risk of bias analyses; exploratory outcomes warrant confirmatory studies; and those outcomes reported without accompanying P values or 95% CIs cannot be evaluated in terms of statistical significance.
Conclusions
SGLT2 inhibitors decreased UACR, reduced the risk for albuminuria onset and progression and promoted albuminuria regression in patients with T2D during long-term follow-up. UACR and albuminuria outcomes with GLP-1 RAs and DPP-4 inhibitors warrant further investigation, as potential drug-specific effects were observed within each class. Available evidence did not show a benefit for GLP-1 RAs and DPP-4 inhibitors in inducing albuminuria regression. Furthermore, SGLT2 inhibitors, but not GLP-1 RAs and DPP-4 inhibitors, potentially improved UACR and albuminuria outcomes in patients with T2D regardless of baseline eGFR and albuminuria status. Short-term (≤ 1 year) effects of novel antidiabetic drugs on changes in UACR and albuminuria remained limited, necessitating further studies. Standardized definitions of UACR and albuminuria outcomes across future studies will allow for the quantitative comparison of treatment effect among novel antidiabetic drug classes in a meta-analysis.
Continuous management of albuminuria with novel antidiabetic drugs may delay CKD onset and slow its progression in patients with T2D. The routine monitoring of UACR or albuminuria changes in clinical practice can inform outcomes with novel antidiabetic drug treatment. The present evidence review suggests that among the novel antidiabetic drugs, SGLT2 inhibitors consistently improved UACR and albuminuria outcomes in patients with T2D, with evidence that continuous treatment could bring about benefit over the long term.
References
Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:2021109119.
Bramlage P, Lanzinger S, van Mark G, et al. Patient and disease characteristics of type-2 diabetes patients with or without chronic kidney disease: an analysis of the German DPV and DIVE databases. Cardiovasc Diabetol. 2019;18(1):33.
Chu L, Fuller M, Jervis K, Ciaccia A, Abitbol A. Prevalence of chronic kidney disease in type 2 diabetes: the Canadian REgistry of Chronic Kidney Disease in Diabetes Outcomes (CREDO) study. Clin Ther. 2021;43(9):1558–73.
Jitraknatee J, Ruengorn C, Nochaiwong S. Prevalence and risk factors of chronic kidney disease among type 2 diabetes patients: a cross-sectional study in primary care practice. Sci Rep. 2020;10(1):6205.
Wu B, Bell K, Stanford A, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns-NHANES 2007–2012. BMJ Open Diabetes Res Care. 2016;4(1): e000154.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150.
Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–8.
Salinero-Fort M, San Andrés-Rebollo FJ, de Burgos-Lunar C, et al. Cardiovascular and all-cause mortality in patients with type 2 diabetes mellitus in the MADIABETES Cohort Study: association with chronic kidney disease. J Diabetes Compl. 2016;30(2):227–36.
Jiang G, Luk AOY, Tam CHT, et al. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with type 2 diabetes. Kidney Int. 2019;95(1):178–87.
Amin AP, Whaley-Connell AT, Li S, Chen SC, McCullough PA, Kosiborod MN. The synergistic relationship between estimated GFR and microalbuminuria in predicting long-term progression to ESRD or death in patients with diabetes: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2013;61(4 Suppl 2):S12-23.
Carrero JJ, Grams ME, Sang Y, et al. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int. 2017;91(1):244–51.
Coresh J, Heerspink HJL, Sang Y, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–27.
Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104.
Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20(5):1069–77.
Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–9.
Jun M, Ohkuma T, Zoungas S, et al. Changes in albuminuria and the risk of major clinical outcomes in diabetes: results from ADVANCE-ON. Diabetes Care. 2018;41(1):163–70.
Keane WF, Brenner BM, de Zeeuw D, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63(4):1499–507.
Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med. 2005;165(8):947–53.
Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28.
Neuen BL, Weldegiorgis M, Herrington WG, Ohkuma T, Smith M, Woodward M. Changes in GFR and albuminuria in routine clinical practice and the risk of kidney disease progression. Am J Kidney Dis. 2021;78(3):350-60.e1.
Norris KC, Smoyer KE, Rolland C, Van der Vaart J, Grubb EB. Albuminuria, serum creatinine, and estimated glomerular filtration rate as predictors of cardio-renal outcomes in patients with type 2 diabetes mellitus and kidney disease: a systematic literature review. BMC Nephrol. 2018;19(1):36.
American Diabetes Association Professional Practice Committee. Chronic kidney disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S175–84.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2019;41(2):255–323.
Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573.
Rangaswami J, Bhalla V, de Boer IH, et al. Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: a scientific statement from the American Heart Association. Circulation. 2020;142(17):e265–86.
Mosenzon O, Del Prato S, Schechter M, et al. From glucose lowering agents to disease/diabetes modifying drugs: a “SIMPLE” approach for the treatment of type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):92.
American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S125–43.
Gallwitz B. Clinical use of DPP-4 inhibitors. Front Endocrinol (Lausanne). 2019;10:389.
Cao H, Liu T, Wang L, Ji Q. Comparative efficacy of novel antidiabetic drugs on cardiovascular and renal outcomes in patients with diabetic kidney disease: a systematic review and network meta-analysis. Diabetes Obes Metab. 2022;24(8):1448–57.
Kanda E, Kashihara N, Matsushita K, et al. Guidelines for clinical evaluation of chronic kidney disease: AMED research on regulatory science of pharmaceuticals and medical devices. Clin Exp Nephrol. 2018;22(6):1446–75.
Heerspink HJL, Greene T, Tighiouart H, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7(2):128–39.
Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84–104.
Chewcharat A, Takkavatakarn K, Isaranuwatchai S, et al. Pleiotropic effects of antidiabetic agents on renal and cardiovascular outcomes: a meta-analysis of randomized controlled trials. Int Urol Nephrol. 2020;52(9):1733–45.
Luo Y, Lu K, Liu G, Wang J, Laurent I, Zhou X. The effects of novel antidiabetic drugs on albuminuria in type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Clin Drug Investig. 2018;38(12):1089–108.
KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–154.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89.
Li J, Woodward M, Perkovic V, et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail. 2020;8(1):57–66.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Neuen BL, Ohkuma T, Neal B, et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS program. J Am Soc Nephrol. 2019;30(11):2229–42.
Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691–704.
Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138(15):1537–50.
Mosenzon O, Wiviott SD, Heerspink HJL, et al. The effect of dapagliflozin on albuminuria in DECLARE-TIMI 58. Diabetes Care. 2021;44(8):1805–15.
Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610–21.
Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(2):356–63.
Mayer GJ, Wanner C, Weir MR, et al. Analysis from the EMPA-REG OUTCOME® trial indicates empagliflozin may assist in preventing the progression of chronic kidney disease in patients with type 2 diabetes irrespective of medications that alter intrarenal hemodynamics. Kidney Int. 2019;96(2):489–04.
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34.
Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907.
Muskiet MHA, Tonneijck L, Huang Y, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(11):859–69.
Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57.
Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–48.
Persson F, Bain SC, Mosenzon O, et al. Changes in albuminuria predict cardiovascular and renal outcomes in type 2 diabetes: a post hoc analysis of the LEADER trial. Diabetes Care. 2021;44(4):1020–6.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–8.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145(8):575–85.
Perkovic V, Toto R, Cooper ME, et al. Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: secondary analysis of the CARMELINA randomized trial. Diabetes Care. 2020;43(8):1803–12.
Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79.
Wanner C, Cooper ME, Johansen OE, et al. Effect of linagliptin versus placebo on cardiovascular and kidney outcomes in nephrotic-range proteinuria and type 2 diabetes: the CARMELINA randomized controlled trial. Clin Kidney J. 2021;14(1):226–36.
Mosenzon O, Leibowitz G, Bhatt DL, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40(1):69–76.
Cornel JH, Bakris GL, Stevens SR, et al. Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care. 2016;39(12):2304–10.
van Ruiten CC, van der Aart-van-der-Beek AB, Richard IJ, et al. Effect of exenatide twice daily and dapagliflozin, alone and in combination, on markers of kidney function in obese patients with type 2 diabetes: a prespecified secondary analysis of a randomized controlled clinical trial. Diabetes Obes Metab. 2021;23(8):1851–8.
Ferreira JP, Zannad F, Butler J, et al. Association of empagliflozin treatment with albuminuria levels in patients with heart failure: a secondary analysis of EMPEROR-Pooled. JAMA Cardiol. 2022;7(11):1148–59.
Idzerda NMA, Clegg LE, Hernandez AF, et al. Prediction and validation of exenatide risk marker effects on progression of renal disease: insights from EXSCEL. Diabetes Obes Metab. 2020;22(5):798–806.
Tonneijck L, Smits MM, Muskiet MH, et al. Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2016;39(11):2042–50.
Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–99.
Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59(9):2036–9.
Jardine M, Zhou Z, Lambers Heerspink HJ, et al. Kidney, cardiovascular, and safety outcomes of canagliflozin according to baseline albuminuria: a CREDENCE secondary analysis. Clin J Am Soc Nephrol. 2021;16(3):384–95.
Jongs N, Greene T, Chertow GM, et al. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(11):755–66.
Jardine MJ, Zhou Z, Mahaffey KW, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020;31(5):1128–39.
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–71.
Cooper ME, Perkovic V, McGill JB, et al. Kidney disease end points in a pooled analysis of individual patient-level data from a large clinical trials program of the dipeptidyl peptidase 4 inhibitor linagliptin in type 2 diabetes. Am J Kidney Dis. 2015;66(3):441–9.
Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42.
Groop PH, Cooper ME, Perkovic V, et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab. 2017;19(11):1610–9.
Acknowledgements
Funding
Sponsorship for this study and Rapid Service Fee were funded by AstraZeneca China.
Medical Writing and Editorial Assistance
Editorial assistance in the preparation of this article was provided by Qing Yun Chong, Tim Stentiford and Abdul Haseeb Syed of Nucleus Global, Shanghai, China, in accordance with Good Publication Practice 3 guidelines. Support for this assistance was funded by AstraZeneca China.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the conceptualization and methodology of the study; the acquisition of data; the analysis and/or interpretation of data; the drafting and reviewing of the manuscript; the critical revision of the manuscript for important intellectual content; and the final approval of the version of the manuscript to be published.
Disclosures
Geng Liu, Xueyu Zhong, Juan Zheng, Jiaoyue Zhang, Wen Kong, Xiang Hu, Jie Min, Wenfang Xia, Tianshu Zeng and Lulu Chen declare that they have no competing interests.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
All data generated or analyzed during this study are included in this published article and supplementary material. Original data are freely available in all electronic databases (references have been given).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, G., Zhong, X., Zheng, J. et al. Comparative Efficacy of Novel Antidiabetic Drugs on Albuminuria Outcomes in Type 2 Diabetes: A Systematic Review. Diabetes Ther 14, 789–822 (2023). https://doi.org/10.1007/s13300-023-01391-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01391-8