Abstract
Introduction
Type 2 diabetes mellitus (T2DM) is often associated with macrovascular complications including cardiovascular diseases (CVDs), resulting in acute coronary syndrome (ACS). Newer potent antiplatelet agents have recently been approved for use in clinical practice. In this analysis, we aimed to systematically compare the cardiovascular outcomes observed with ticagrelor versus clopidogrel in T2DM patients with ACS.
Methods
From August to September 2022, electronic databases were searched for publications that compared cardiovascular outcomes observed with ticagrelor versus clopidogrel in patients with T2DM. The statistical analysis was carried out using RevMan 5.4 software. A random effect statistical model was used to analyze the data. Risk ratios (RR) with 95% confidence intervals (CIs) were used to represent the data post analysis.
Results
A total of 5868 participants with T2DM were included in this analysis, of which 1944 participants were assigned to the ticagrelor group and 3924 participants were assigned to the clopidogrel group. Our analysis showed that ticagrelor was associated with a significantly lower risk of major adverse cardiac events (MACEs) (RR: 0.64, 95% CI: 0.49–0.84; P = 0.001), all-cause mortality (RR: 0.65, 95% CI: 0.51–0.83; P = 0.0004), and cardiac death (RR: 0.60, 95% CI: 0.43–0.84; P = 0.003) in comparison to clopidogrel. However, the risks of repeated revascularization (RR: 1.48, 95% CI: 0.44–4.99; P = 0.53), stent thrombosis (RR: 0.70, 95% CI: 0.18–2.71; P = 0.60), reinfarction (RR: 0.85, 95% CI: 0.58–1.23; P = 0.39), and stroke (RR: 0.56, 95% CI: 0.14–2.21; P = 0.41) were similar. Ticagrelor was associated with a significantly higher risk of minor bleeding (RR: 1.53, 95% CI: 1.07–2.19; P = 0.02), whereas the risk for major bleeding (RR: 1.08, 95% CI: 0.55–2.10; P = 0.82) was not significantly different.
Conclusions
In these T2DM patients with ACS, a significantly lower risk of major adverse cardiovascular events including all-cause mortality was observed in the ticagrelor group compared with the clopidogrel group. However, T2DM patients who were assigned to ticagrelor showed a significantly higher minor bleeding risk. Larger clinical trials should be able to confirm these hypotheses.
Plain Language Summary
Type 2 diabetes mellitus (T2DM) is increasing all over the world, and is often associated with macrovascular complications including cardiovascular diseases (CVDs), leading to acute coronary syndrome (ACS).
Dual antiplatelet therapy (DAPT) with aspirin and clopidogrel is recommended in these patients.
However, due to clopidogrel hyporesponsiveness, especially in patients with T2DM, a more potent antiplatelet is needed.
Recently, newer, more potent antiplatelet agents have been approved for use in clinical practice.
A significantly lower risk of major adverse cardiovascular outcomes including all-cause mortality was observed with ticagrelor compared with clopidogrel in these patients with T2DM.
However, ticagrelor was associated with a significantly higher minor bleeding risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Type 2 diabetes mellitus (T2DM) is increasing all over the world, and is often associated with macrovascular complications including cardiovascular diseases (CVDs), leading to acute coronary syndrome (ACS) |
Dual antiplatelet therapy (DAPT) with aspirin and clopidogrel is recommended in these patients |
However, due to clopidogrel hyporesponsiveness, especially in patients with T2DM, a more potent antiplatelet is needed |
Recently, newer, more potent antiplatelet agents have been approved for use in clinical practice |
A significantly lower risk of major adverse cardiovascular outcomes including all-cause mortality was observed with ticagrelor compared to clopidogrel in these patients with T2DM |
However, ticagrelor was associated with a significantly higher minor bleeding risk |
Introduction
Type 2 diabetes mellitus (T2DM) is rising worldwide, and is often associated with macrovascular complications including cardiovascular diseases (CVDs), which could lead to acute coronary syndrome (ACS) [1, 2]. Following percutaneous coronary intervention (PCI) in these patients, guidelines have recommended dual antiplatelet therapy (DAPT) with aspirin and clopidogrel [3]. However, due to clopidogrel hyporesponsiveness [4], especially in patients with T2DM, which is often associated with platelet hyperactivity [5], a more potent antiplatelet is needed [6]. Recently, new potent antiplatelet agents including ticagrelor and prasugrel have been approved for use in clinical practice [7]. Ticagrelor has recently been prescribed to patients with T2DM and coexisting CVDs [8]. However, ticagrelor has seldom been systematically compared with clopidogrel through a meta-analysis in patients with T2DM. In this analysis, we aimed to systematically compare the cardiovascular outcomes observed with ticagrelor versus clopidogrel in T2DM patients with ACS.
Methods
Search Databases
Databases including MEDLINE (subset PubMed), EMBASE, http://www.ClinicalTrials.gov, Web of Science, Mendeley, Google scholar. and the Cochrane database were searched from August to the end of September 2022 for publications that compared cardiovascular outcomes between ticagrelor and clopidogrel in T2DM patients with ACS. Reference lists of relevant publications were also checked for suitable articles.
Search Terms and Search Strategies
The following search terms or phrases were used during the search process:
“ticagrelor, clopidogrel, and diabetes mellitus”;
“ticagrelor, clopidogrel, and type 2 diabetes mellitus”;
“ticagrelor, clopidogrel, and T2DM”;
“ticagrelor, clopidogrel, diabetes mellitus, and percutaneous coronary intervention”;
“ticagrelor, clopidogrel, diabetes mellitus, and PCI”;
“ticagrelor, clopidogrel, diabetes mellitus, and coronary revascularization”;
“ticagrelor, clopidogrel, diabetes mellitus, and acute coronary syndrome”;
“ticagrelor, clopidogrel, diabetes mellitus, and STEMI”;
“ticagrelor, clopidogrel, diabetes mellitus, and ACS’’.
Only English publications were considered for this paper.
Inclusion and Exclusion Criteria
Studies were included if:
-
(a)
they were randomized trials or observational studies comparing the cardiovascular outcomes in patients with T2DM treated by ticagrelor versus clopidogrel for ACS or following PCI;
-
(b)
they were published in English;
-
(c)
they reported dichotomous variables.
Studies were excluded if:
-
(a)
they were meta-analyses, systematic reviews, letter to editors, case studies, or literature reviews;
-
(b)
they did not involve patients with diabetes mellitus;
-
(c)
they did not report cardiovascular outcomes;
-
(d)
they were published in another language apart from English;
-
(e)
they consisted of continuous variables;
-
(f)
they were duplicated studies.
Outcomes and Definitions
The cardiovascular outcomes that were reported in the original studies have been listed in Table 1.
The following endpoints were assessed in this analysis:
-
(a)
Major adverse cardiovascular events (MACEs) consisting of the composite endpoints including mortality, myocardial infarction, and revascularization; and when stroke was included, this composite endpoint was referred to as major adverse cardiovascular and cerebrovascular events (MACCEs);
-
(b)
all-cause mortality;
-
(c)
cardiac death;
-
(d)
repeated revascularization;
-
(e)
stent thrombosis;
-
(f)
reinfarction;
-
(g)
stroke;
-
(h)
major bleeding: any major bleeding reported including thrombolysis in myocardial infarction (TIMI) defined major bleeding [9] and bleeding defined according to the academic research consortium (BARC), bleeding [9] type 3–5.
-
(i)
Minor bleeding: any minor bleeding reported including TIMI defined minor bleeding and BARC bleeding type 1 and 2.
Data Extraction and Quality Assessment
Two authors independently extracted data from the selected original studies. Data included authors’ names; year of publication; participants’ enrollment time; type of studies; total number of T2DM participants who were assigned to the ticagrelor versus the clopidogrel group, respectively; the type of coronary artery disease; the number of events associated with each endpoint; baseline features including age, gender, comorbidities such as hypertension, dyslipidemia, smoking history, and oral antihyperglycemic agents, as well as the percentage of participants on insulin therapy. Data were carefully extracted and cross-checked by the two authors. Any disagreement that occurred during this data extraction process was discussed with the corresponding author who was responsible for making the final decision.
The methodological assessment of the randomized trials was carried out using the Cochrane tool [10], while the methodological quality of the observational studies was carried out using the Newcastle Ottawa Scale (NOS) [11]. Grades from A to C were allotted to the studies denoting low to high risk of bias.
Statistical Analysis
The statistical analysis was carried out by the new version of the RevMan software, version 5.4. Risk ratios (RR) with 95% confidence intervals (CIs) were used to represent the data post analysis. A random effect statistical model was used to analyze the data. Heterogeneity was assessed by the Q statistic test, whereby a P-value ≤ 0.05 was considered statistically significant. Heterogeneity was also assessed by the I2 test, whereby a lower I2 percentage denoted lower heterogeneity, and a higher I2 value denoted increasing heterogeneity.
Sensitivity analysis was carried out by an exclusion method, whereby each original study included in the final analysis was excluded one by one, and a new analysis was carried out each time and compared with the main result of this analysis for any statistical difference.
This meta-analysis included less than ten studies,therefore, publication bias was visually assessed through funnel plots.
Compliance with Ethical Guidelines
This study is a meta-analysis, and does not involve experiment on humans or animals carried out by any of the authors. Hence, ethical or board review approval was not required. Data were extracted from previously published original studies.
Results
Search Outcomes
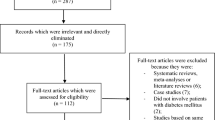
The preferred reporting items in systematic reviews and meta-analyses (PRISMA) guideline [12] was followed. Our search resulted in a total of 136 publications. The authors carefully assessed the abstracts and titles. Following this initial assessment, 106 publications were eliminated since they were not based on the scope and idea of this research paper. Therefore, 30 full text articles were assessed for eligibility.
Another assessment of the full text articles was carried out. Based on this second assessment, elimination was carried out based on the criteria for inclusion and exclusion. Publications were eliminated because they did not compare the outcomes in patients with T2DM who were assigned to either ticagrelor or clopidogrel, but instead, compared outcomes in T2DM and non-diabetes mellitus, they reported platelet aggregations as endpoints that did not satisfy the inclusion and exclusion criteria of this research, or they were duplicated studies.
Finally, seven studies [13,14,15,16,17,18,19] were included in this analysis, as shown in Fig. 1.
General and Baseline Features of the Studies
The general features of the studies are listed in Table 2. A total of 5868 participants with T2DM were included in this analysis, of which 1944 participants were assigned to the ticagrelor group and 3924 participants were assigned to the clopidogrel group. Patients with ACS were included in this analysis. The studies reported a follow-up time period ranging from 6 to 24 months.
The baseline characteristics of the participants are listed in Table 3. Mean age varied from 47.9 to 79.1 years, with the number of male participants ranging from 29.3% to 78.9% depending on the study. Hypertension (26.0–74.5%), dyslipidemia (12.6–68.4%), smokers (21.3–57.1%), and participants on insulin therapy (5.40–39.1%) or oral antihyperglycemic agents (33.1–85.0%) are also reported in Table 3.
Main Results of this Analysis
Our analysis showed that ticagrelor was associated with a significantly lower risk of MACEs (RR: 0.64, 95% CI: 0.49–0.84; P = 0.001), all-cause mortality (RR: 0.65, 95% CI: 0.51–0.83; P = 0.0004), and cardiac death (RR: 0.60, 95% CI: 0.43–0.84; P = 0.003) in comparison with clopidogrel in these patients with T2DM, as shown in Fig. 2. However, the risks of repeated revascularization (RR: 1.48, 95% CI: 0.44–4.99; P = 0.53), stent thrombosis (RR: 0.70, 95% CI: 0.18–2.71; P = 0.60), reinfarction (RR: 0.85, 95% CI: 0.58–1.23; P = 0.39), and stroke (RR: 0.56, 95% CI: 0.14–2.21; P = 0.41) were similarly manifested, as shown in Fig. 2.
When bleeding risks were assessed, ticagrelor was associated with a significantly higher risk of minor bleeding (RR: 1.53, 95% CI: 1.07–2.19; P = 0.02) as shown in Fig. 3, whereas the risk of major bleeding (RR: 1.08, 95% CI: 0.55–2.10; P = 0.82) was not significantly different with ticagrelor versus clopidogrel.
The results of this analysis are summarized in Table 4.
Sensitivity Analysis and Publication Bias
When the sensitivity analysis was carried out, consistent results were obtained throughout. When each study were excluded one at a time and a new analysis was carried out, the obtained result was not significantly different from the main results of this analysis. In addition, a simple analysis of funnel plots provided a vital test for the likely presence of bias in the meta-analyses. The funnel plot, which is a plot of effect estimates against sample size, could be visually assessed for asymmetrical findings that could predict discordance of results when meta-analyses are compared with larger trials. Therefore, by visually assessing the symmetry of the funnel plot (Fig. 4), we could confirm a low evidence of publication bias across the studies that assessed all the cardiovascular outcomes in these patients with T2DM. Publication bias is represented in Fig. 4.
Discussion
In this meta-analysis, we compared the cardiovascular outcomes observed in T2DM patients with ACS who were assigned to a ticagrelor group versus those who were assigned to a clopidogrel group. Our results showed that ticagrelor was associated with a significantly lower risk of MACEs and mortality. However, the risk of minor bleeding was significantly higher with ticagrelor in comparison to clopidogrel in these patients with T2DM. Nevertheless, significant major bleeding was not observed with ticagrelor.
Similar to this analysis, a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial [20] that assessed ticagrelor versus clopidogrel in patients with ACS and diabetes mellitus showed the former to reduce ischemic events in ACS patients irrespective of diabetic status and glycemic control, without increasing the risk of major bleeding. It should be noted that this PLATO substudy included 4662 participants with T2DM, of which 1036 were on insulin therapy.
A post hoc analysis of the ad hoc PCI study [21], which was a prospective, open-label, randomized, multicenter, parallel-group, phase 4 pharmaco-dynamic study performed at 15 centers in the USA and involving patients with diabetes mellitus, showed that compared with clopidogrel, faster and enhanced platelet inhibition was achieved with ticagrelor. Similarly, in the OPTIMUS-6 study [22], which analyzed the pharmacodynamic and pharmacokinetic effects of a low maintenance dose ticagrelor regimen versus standard dose clopidogrel in diabetes mellitus patients without previous MACEs undergoing elective PCI, the authors showed that a 60 mg twice daily dose of ticagrelor minimum dose regimen achieved better and more sustained platelet inhibition compared with the standard dose clopidogrel. The results were in favor of ticagrelor even in the Clopidogrel High Dose Versus Ticagrelor for Antiplatelet Maintenance in Diabetic Patients (CLOTILDIA) study [23]. The faster and more potent antithrombotic property of ticagrelor in patients with T2DM were further demonstrated in a study published by Zafar et al. [24].
Our results showed a significant decrease in MACEs with ticagrelor compared with clopidogrel in patients with T2DM. Current guidelines issued by the American Heart Association/American College of Cardiology recommend ticagrelor over clopidogrel for DAPT with aspirin in patients with ACS. The potent effect of ticagrelor could be because it works differently to clopidogrel. Even though it inhibits adenosine-5 diphosphate (ADP), which plays an important role in blood clotting, it does this by reversibly binding to the receptor P2Y12 on the surface of platelets [25]. Reversible binding means platelet activities could be restored at a later time once the concentration of ticagrelor decreases to a certain level. In contrast, clopidogrel binds irreversibly to the P2Y12 receptors of platelet surfaces, blocking platelets from aggregating for the remainder of their lifespan (about 10 days). Moreover, ticagrelor could work quicker than clopidogrel. Maximum platelet inhibition with ticagrelor could be reached within 2 h in comparison with clopidogrel, which could take over 8 h. Also, clopidogrel is a prodrug, meaning that it requires conversion in the liver to its active form before it can work. However, ticagrelor is not a prodrug, therefore it has a quicker onset of action.
Our current analysis was based on diabetes mellitus patients with ACS and in this category of patients, the results showed ticagrelor to be associated with a significantly lower risk of MACEs, but a significantly higher risk of minor bleeding in comparison to clopidogrel. However, in diabetes patients with stable coronary artery disease, ticagrelor in combination with aspirin was associated with a higher incidence of major bleeding [26].
Limitations
Similar to other studies, this analysis also limitations. First, due to the limited number of participants, the results might not be robust. Another limitation is that several clinical outcomes, such as dyspnea, heart failure, rehospitalization, gastrointestinal bleeding, intracranial bleeding, and so on, could not be analyzed because they were reported in only one study. Another limitation is that in one study, patients in the experimental group were assigned to both ticagrelor and prasugrel instead of ticagrelor alone. This might have a minor impact on the results. Moreover, in two studies, the follow-up time period was not mentioned, whereas in other studies where the follow-up time period was stated, and varied among studies. Another limitation is that the duration of diabetes mellitus, the cardiac medications used by the patients, and the percentage of patients with T2DM on insulin therapy or on oral antihyperglycemic agents were not taken into consideration. Also, in most of the original studies, the glycosylated hemoglobin was not reported for the patients, so it was not known whether blood sugar in those participants was well controlled or uncontrolled.
Conclusions
In these patients with T2DM, a significantly lower risk of major adverse cardiovascular events including all-cause mortality was observed in the ticagrelor group compared with the clopidogrel group. However, T2DM patients who were assigned to the ticagrelor group showed a significantly higher minor bleeding risk. Larger clinical trials should be able to confirm these hypotheses.
References
Viigimaa M, Sachinidis A, Toumpourleka M, Koutsampasopoulos K, Alliksoo S, Titma T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18(2):110–6.
Katsiki N, Papanas N. Diabetes Mellitus and Acute Coronary Syndrome: A Lethal Combination Requiring Better Therapeutic Strategies. Curr Vasc Pharmacol. 2020;18(1):77–9.
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123–55.
Dharam JK, Steven PM, Carlos AA, Darren KM. State-of-the-Art: Hypo-responsiveness to Oral Antiplatelet Therapy in Patients with Type 2 Diabetes Mellitus. Curr Cardiovasc Risk Rep. 2015;9:4.
Arun N, Azfar GZ, Sally MM. Platelet Hyperactivity in Type 2 Diabetes: Role of Antiplatelet Agents. Diab Vasc Dis Res. 2008;5(2):138–44.
Chadi D, Elias BH, Mazen SA-F. A New Era for Antiplatelet Therapy in Patients with Acute Coronary Syndrome. Am J Med Sci. 2010;340(5):407–11.
Schüpke S, Neumann F-J, Menichelli M, et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2019;381(16):1524–34.
Ndrepepa G, Kastrati A, Menichelli M, et al. Ticagrelor or Prasugrel in Patients With Acute Coronary Syndromes and Diabetes Mellitus. JACC Cardiovasc Interv. 2020;13(19):2238–47.
Quinlan DJ, Eikelboom JW, Goodman SG, Welsh RC, Fitchett DH, Théroux P, Mehta SR. Implications of variability in definition and reporting of major bleeding in randomized trials of oral P2Y12 inhibitors for acute coronary syndromes. Eur Heart J. 2011;32(18):2256–65.
Wouter JK, Nan van G, Mariet van der HL, et al. The Prognostic Value of Bleeding Academic Research Consortium (BARC)-Defined Bleeding Complications in ST-Segment Elevation Myocardial Infarction: A Comparison with the TIMI (Thrombolysis In Myocardial Infarction), GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries), and ISTH (International Society on Thrombosis and Haemostasis) bleeding classifications. J Am Coll Cardiol. 2014;63(18):1866–75.
Julian PTH, Douglas GA, Peter CG et al. The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 2011;343:d5928.
Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol. 2010;25(9):603–5.
Page MJ, McKenzie JE, Bossuyt PM, The PRISMA Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2020;2021(372): n71.
Kye TA, Seok-Woo S, Ung LC et al. Comparison of 1-Year Clinical Outcomes between Prasugrel and Ticagrelor versus Clopidogrel in Type 2 Diabetes Patients with Acute Myocardial Infarction underwent Successful Percutaneous Coronary Intervention. Medicine (Baltimore). 2019;98(11):e14833.
He P, Luo X, Li J, et al. Clinical Outcome between Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndrome and Diabetes. Cardiovasc Ther. 2021;2021:5546260.
Li D-T, Li S-B, Zheng J-Y, et al. Analysis of Ticagrelor’s Cardio-protective Effects on Patients with ST-Segment Elevation Acute Coronary Syndrome Accompanied with Diabetes. Open Med (Wars). 2019;14:234–40.
Liu Y, Ding L-Y, Li X-Z. Therapy with Ticagrelor for ST-Elevated Acute Coronary Syndrome Accompanied by Diabetes Mellitus. Eur Rev Med Pharmacol Sci. 2019;23(3 Suppl):312–8.
Liu Z, Tian R, Wang Y, et al. Platelet Inhibition with Ticagrelor versus Clopidogrel in Diabetic Patients after Percutaneous Coronary Intervention for Chronic Coronary Syndromes. Thromb Haemost. 2020;120(8):1221–9.
Mina WM, Mohamed AE, Hala FZ, Hanan SE-A. Diabetes and CYP2C19 Polymorphism Synergistically Impair the Antiplatelet Activity of Clopidogrel Compared with Ticagrelor in Percutaneous Coronary Intervention-Treated Acute Coronary Syndrome Patients. J Cardiovasc Pharmacol. 2020;76(4):478–488.
Wang C-A, Hsieh Y-C, Huang C-Y, et al. Comparison between Ticagrelor versus Clopidogrel in Long Term Outcomes of Taiwanese Diabetic Subjects with Acute Coronary Syndrome Undergoing Successful Revascularization: From TSOC ACS-DM Registry. Medicine (Baltimore). 2020;99(19): e19969.
Stefan J, Dominick JA, Jan HC et al. Ticagrelor vs. Clopidogrel in Patients with Acute Coronary Syndromes and Diabetes: A Substudy from the PLATelet Inhibition and Patient Outcomes (PLATO) Trial. Eur Heart J. 2010;31(24):3006–16.
Joseph MS, Dominick JA, Francesco F et al. Impact of Diabetes Mellitus on the Pharmacodynamic Effects of Ticagrelor versus Clopidogrel in Troponin-Negative Acute Coronary Syndrome Patients Undergoing Ad Hoc Percutaneous Coronary Intervention. J Am Heart Assoc. 2017;6(4):e005650.
Franchi F, Rollini F, Been L, et al. Pharmacodynamic and Pharmacokinetic Effects of a Low Maintenance Dose Ticagrelor Regimen versus Standard Dose Clopidogrel in Diabetes Mellitus Patients Without Previous Major Cardiovascular Events Undergoing Elective Percutaneous Coronary Intervention: The OPTIMUS-6 Study. Circulation. 2020;142(15):1500–2.
Mangiacapra F, Panaioli E, Colaiori I, et al. Clopidogrel versus Ticagrelor for Antiplatelet Maintenance in Diabetic Patients Treated with Percutaneous Coronary Intervention: Results of the CLOTILDIA Study (Clopidogrel High Dose versus Ticagrelor for Antiplatelet Maintenance in Diabetic Patients). Circulation. 2016;134(11):835–7.
Zafar MU, Baber U, Smith DA, et al. Antithrombotic Potency of Ticagrelor versus Clopidogrel in Type-2 Diabetic Patients with Cardiovascular Disease. Thromb Haemost. 2017;117(10):1981–8.
Tantry US, Bonello L, Aradi D, et al. Consensus and Update on the Definition of on-Treatment Platelet Reactivity to Adenosine Diphosphate Associated with Ischemia and Bleeding. J Am Coll Cardiol. 2013;62(24):2261–73.
Steg PG, Bhatt DL, Simon T, et al. Ticagrelor in Patients with Stable Coronary Disease and Diabetes. N Engl J Med. 2019;381(14):1309–20.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
The authors ZJ, LL and PKB were responsible for the conception and design, acquisition of data, analysis and interpretation of data, drafting the initial manuscript and revising it critically for important intellectual content. The author ZJ wrote the final paper. All the authors agreed to and approved the manuscript as it is.
Disclosures
The authors Zhiming Jiang, Le Liu and Pravesh Kumar Bundhun declare that they have no competing interests.
Compliance with Ethics Guidelines
This meta-analysis is based on previously conducted studies and does not contain any new study with human participants or animals performed by any of the authors. Therefore, an ethical approval was not required.
Data Availability
All data generated or analyzed during this study are included in this published article. References of the original papers involving the data source which have been used in this paper have been listed in the main text of this current manuscript. All data are publicly available in electronic databases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jiang, Z., Liu, L. & Bundhun, P.K. Cardiovascular Outcomes Observed with Ticagrelor versus Clopidogrel in Type 2 Diabetes Mellitus Patients with Acute Coronary Syndrome: A Meta-analysis. Diabetes Ther 14, 387–399 (2023). https://doi.org/10.1007/s13300-022-01354-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01354-5