Abstract
Objective
This study aimed to evaluate the long-term cost-effectiveness of once-weekly subcutaneous semaglutide versus polyethylene glycol loxenatide (PEG-loxenatide) in patients with type 2 diabetes uncontrolled on metformin, from a Chinese healthcare systems perspective.
Methods
The study applied the Swedish Institute of Health Economics Diabetes Cohort Model to evaluate the long-term clinical and economic outcomes of once-weekly treatment of semaglutide at 0.5 mg and 1.0 mg, respectively, versus PEG-loxenatide 0.2 mg, over a 40-year time horizon. Baseline cohort characteristics were collected from the SUSTAIN China trial. A network meta-analysis was conducted to obtain comparative treatment effects of once-weekly semaglutide and PEG-loxenatide based on two phase 3a clinical trials. Drug costs were sourced from the national bidding price of China. Outcomes were discounted at 5.0% per annum. One-way sensitivity analysis and probabilistic sensitivity analysis were conducted to assess the uncertainty of the base-case results.
Results
When compared with PEG-loxenatide 0.2 mg, the projections of outcomes over the 40-year time horizon in patients with type 2 diabetes uncontrolled on metformin showed that treatment with once-weekly semaglutide 0.5 mg and 1.0 mg were associated with improved discounted life expectancy by 0.08 and 0.12 years, and improved discounted quality-adjusted life expectancy by 0.16 and 0.22 quality-adjusted life-years, respectively. Once-weekly semaglutide 0.5 mg and 1.0 mg were achieved at lifetime cost savings of 19,309 China Yuan (CNY) and 10,179 CNY, respectively. Sensitivity analyses verified the robustness of the results.
Conclusion
From the perspective of Chinese healthcare systems, treatment with once-weekly subcutaneous semaglutide represents a dominant option versus PEG-loxenatide for patients with type 2 diabetes uncontrolled on metformin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Type 2 diabetes causes heavy economic and clinical burdens in China. |
Once-weekly semaglutide is a novel glucagon-like peptide-1 receptor agonist (GLP-1 RA), and its clinical benefit have been confirmed in the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) trials. |
Once-weekly semaglutide has been shown to be more cost-effective than dulaglutide in the Chinese setting, but a direct cost-effectiveness comparison between once-weekly semaglutide and the locally preferred once-weekly GLP-1 RA polyethylene glycol loxenatide (PEG-loxenatide) is missing. |
What was learned from the study? |
Once-weekly semaglutide 0.5 mg and 1.0 mg are cost-saving treatments compared with PEG-loxenatide 0.2 mg in China. |
This study highlights the long-term clinical and economic value of once-weekly semaglutide for the treatment of type 2 diabetes patients in China. |
Introduction
Diabetes is one of the most severe threats to global health. In China, diabetes currently affects about 129 million people [1], of whom about 90% have type 2 diabetes (T2D) [2]. However, that of all the patients in China with diabetes, only 36.7% were diagnosed, 32.9% received treatment, and 16.5% achieved the glycemic control target [3]. The International Diabetes Federation estimated that China's total diabetes-related health expenditure was USD 109.0 billion in 2019 [4], which represents 11.4% of all medical costs in 2019 [5]. Chronic diabetic complications, in particular cardiovascular complications, have been shown to cause most of diabetes-related expenditures [6]. Shen et al. [7] used electronic health records to analyze the components of diabetes-related costs (including costs of antidiabetics and of treating complications) in China, and found that of the total expenditure related to diabetes, 54% was spent on treating the cardiovascular complications of individuals with diabetes. Therefore, it is important that a greater proportion of patients with diabetes achieve glycemic control and multifactorial treatment targets, thereby improving long-term health outcomes and reducing the costs of treating diabetes-related complications both for patients and Chinese society as a whole.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are a class of interventions for T2D associated with improved glycemic control, weight loss benefits and reduced hypoglycemia risk. In addition, some recent cardiovascular outcomes trials (CVOTs) have indicated that several long-acting human GLP-1 RAs are associated with improved cardiovascular outcomes in patients with T2D [8,9,10,11]. In China, the latest guideline for type 2 diabetes in China, published by the Chinese Diabetes Society in 2020, recommends that patients with T2D with inadequate glycemic control on metformin treatment and lifestyle intervention are to be treated with GLP-1RAs to improve their glycemic control [2]. In particular, the guideline recommends that GLP-1-RAs in combination with metformin should used for patients with atherosclerotic cardiovascular disease (ASCVD) and/or with high risk of cardiovascular and cerebrovascular disease (CVD), regardless of whether glycated hemoglobin A1c (HbA1c) has reached the target, to improve the CVD outcomes for patients if there is no contraindication [2].
Semaglutide is a novel long-acting, human-based GLP-1RA that has been developed for the treatment of T2D. Semaglutide has 94% structural homology to native human GLP-1. Three minor but important modifications significantly extend the half-life of semaglutide to 165 h and make it suitable to be administered on a once-weekly schedule: amino acid substitutions at position 8 (alanine to alpha-aminoisobutyric acid a synthetic amino acid) and at position 34 (lysine to arginine), and acylation of the peptide backbone with a spacer and C-18 fatty di-acid chain to lysine at position 26 [12, 13]. Throughout the global Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) trials, compared to all the other comparators once-weekly semaglutide displayed greater short-term efficacy, with more significant reductions in HbA1c and multiple cardiometabolic benefits (including improving the control of blood pressure, blood lipids and body weight, among others) [14,15,16,17,18,19,20,21,22]. Also, the SUSTAIN China trial showed that the proportion of patients achieving the HbA1c target (HbA1c < 7%) with once-weekly semaglutide was as high as 86.1% in the Chinese diabetes population [23].
In addition to clinical considerations, economic value assessment of a novel intervention is also important for healthcare decisions given the enormous disease burden of diabetes. In China, three once-weekly GLP-1 RAs have been included in the National Reimbursement Drug List, including once-weekly semaglutide (Ozempic®; Novo Nordisk, Bagsværd, Denmark), dulaglutide (Trulicity®; Eli Lilly, Indianapolis, IN, USA), and polyethylene glycol loxenatide (PEG-loxenatide; Fu Laimei®; Hansoh Pharma, Lianyungang, China). The long-term cost-effectiveness of once-weekly semaglutide compared with dulaglutide in the Chinese setting was evaluated in our previous study [24]. However, to date there has been no cost-effectiveness comparison between once-weekly semaglutide and PEG-loxenatide. Semaglutide is available in two treatment doses of 0.5 mg and 1.0 mg, administered once-weekly; PEG-loxenatide is also available in two treatment doses of 0.1 mg and 0.2 mg, administered once-weekly [25]. Because PEG-loxenatide 0.1 mg is rarely used in clinical practice in China, we did not include this dose in our study.
Thus, this study aimed to evaluate the long-term cost-effectiveness of once-weekly semaglutide 0.5 mg and 1.0 mg versus PEG-loxenatide 0.2 mg for the treatment of people with T2D who were not controlled with metformin from the perspective of Chinese healthcare systems.
Methods
Model Approach
For the purpose of this cost-effectiveness analysis, we have attempted to use a diabetes model that is easy to use, transparent, and able to conduct uncertainty analysis since these qualities were considered appropriate and expected by Chinese stakeholders. The Swedish Institute of Health Economics Diabetes Cohort Model (IHE-DCM) (version 4.4.2) was used for this study, as it meets these criteria. In recent years, the IHE-DCM has been increasingly applied to health technology assessments of hypoglycemic drugs. The model was designed in Microsoft® Excel (Microsoft Corp., Redmond, WA, USA) using Visual Basic for Applications (VBA). It can be run with one intervention and up to 12 comparator arms, allowing for simultaneous comparison of multiple treatment strategies. The cycle length is 1 year, and the maximum time horizon is 40 years. The IHE-DCM can simulate the occurrence of diabetes-related complications by constructing macrovascular and microvascular Markov sub-models, respectively. The macrovascular Markov chain consists of combinations of stages of ischemic heart disease, myocardial infarction, stroke, and heart failure. The microvascular Markov chain consists of combinations of stages of eye disease, kidney disease, and lower extremity disease. The authors accessed and used the IHE-DCM through a user agreement with the Swedish Institute for Health Economics (Lund, Sweden).
The IHE-DCM projects long-term outcomes based on user inputs and user-defined selection of risk equations. User inputs comprise cohort baseline characteristics, treatment algorithms and clinical effects, cost, and health utility. The mortality risk equations are sourced from the UK Prospective Diabetes Study (UKPDS) 68 [26] or UKPDS 82 [27]. Users can choose four sets of macrovascular risk equations among UKPDS 68 [26], UKPDS 82 [27], the Swedish National Diabetes Register (NDR) [28], or the Australian Fremantle Diabetes Study (FDS) [29]. Users do not need to select the microvascular risk equation because there is only one set of risk equations in the model [30,31,32]. Outputs of the IHE-DCM include cumulative incidence of macro- and microvascular complications, life expectancy, quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratio (ICER).
For the whole analysis, the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist was followed [33].
Discount and Time Horizon
A 40-year time horizon was applied in this study. The projected clinical and economic outcomes were discounted at a rate of 5.0% annually, which is in line with the guideline of cost-effectiveness analysis in China [34].
Baseline Cohort Characteristics
The target population of this study was assumed to be a cohort of patients with T2D uncontrolled with metformin in China. Baseline demographic characteristics, baseline HbA1c, body mass index (BMI), and estimated glomerular filtration rate were derived from the SUSTAIN China trial [23]. The SUSTAIN China study was a 30-week, randomized, double-blind, multicenter clinical trial with the aim to evaluate the efficacy and safety of once-weekly semaglutide in patients with T2D inadequately controlled on metformin treatment. Participants' mean age at baseline was 52.27 years; the mean duration of diabetes was 6.07 years; and the proportion of women was 39.50%. Other baseline biochemical parameters and baseline risk of diabetes-related microvascular and macrovascular complications were mainly sourced from another study of Chinese patients with T2D [35] (see Electronic Supplementary Material [ESM] Table 1).
Treatment Algorithm and Treatment Effects
In the base-case simulation, patients initiate a 3-year treatment of once-weekly semaglutide (0.5 or 1.0 mg) or PEG-loxenatide (0.2 mg). The treatment period is consistent with previously published cost-effectiveness studies [36,37,38]. It was assumed that the GLP-1RA treatment effect would last for 3 years, following which treatment with once-weekly semaglutide and PEG-loxenatide would cease and treatment with basal insulin (insulin glargine) would be initiated and used for the remainder of the patient's lifetime.
A network meta-analysis (NMA) was conducted to compare the efficacy and safety of once-weekly semaglutide and PEG-loxenatide by R 4.2.0 software ® Foundation for Statistical Computing, Vienna, Austria) since there is no head-to-head clinical trial. This NMA was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [39]. The NMA showed that once-weekly semaglutide 0.5 and 1.0 mg were associated with greater reductions in HbA1c, BMI, and systolic blood pressure (SBP) and greater improvements in blood lipid levels, compared with PEG-loxenatide 0.2 mg. Further details on this NMA are provided in ESM Tables 2, 3; ESM Fig. 1, including the search strategy, study selection, and results of the difference in treatment effect between once-weekly semaglutide and PEG-loxenatide.
In the cost-effectiveness analysis, the treatment effects on HbA1c, BMI, SBP, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride for patients treated with once-weekly semaglutide and PEG-loxenatide were obtained from the NMA. The cardiovascular protection effect of once-weekly semaglutide was collected from the cardiovascular outcome of SUSTAIN 6 [9]. It was assumed that PEG-loxenatide has no cardiovascular protective effect since there was no evidence to show the cardiovascular benefit of PEG-loxenatide. Rates of hypoglycemia events were taken from their respective clinical trials [14, 25]. All of the treatment effects and adverse event rates are summarized in Table 1.
Long-term Parameter Progression
According to the study of Kahn et al. [40], it was assumed that HbA1c would drift upwards at a rate of 0.14% per year. Other biomarkers, such as SBP and lipid levels, were assumed to remain at the same level as when GLP-1 RA treatment was discontinued. Risk equations sourced from the NDR were employed to predict the incidence of macrovascular complications because they could provide the best fit for macrovascular outcomes when compared with UKPDS 68 and UKPDS 82 [41]. UKPDS 82 was used to predict the incidence of mortality.
Costs and Utilities
From the perspective of Chinese healthcare systems, this study captured direct medical costs (including medication costs, treatment costs associated with diabetes-related complications and hypoglycemic events) in 2021 Chinese Yuan (CNY).
Medication costs were obtained from the national average bidding price in June 2022. The dosage of insulin glargine U100 was based on a meta-analysis evaluating the daily dosage of basal insulin among Chinese patients with T2D [42]. It was assumed that treatment adherence for each intervention was 100%. Annual costs also captured concomitant medication (i.e., metformin) and needle use. According to the assumption that there was no difference in the frequency of self-monitoring of blood glucose (SMBG) between GLP-1 RA treatments, the cost of SMBG was not included. Annual medication costs are given in ESM Table 4.
The costs of diabetes-related complications were obtained from published literature [35, 43,44,45,46,47,48]. The costs from the literature were inflated to 2021 CNY through China's healthcare consumer price index (ESM Table 5).
The utility of the patient at baseline and the disutility of complications and demographic factors were taken from published literature [47, 49,50,51,52] and are given in ESM Table 6.
Sensitivity Analyses
As the extrapolation of outcomes over patients’ lifetimes from short-term clinical data is associated with uncertainty, sensitivity analyses were performed on key assumptions and key parameters to test the robustness of the base-case analysis. Variations included in the one-way sensitivity analyses were: (1) shortening the time horizon to 20 and 30 years, respectively; (2) applying discount rates of 0%, 3% and 8% for clinical and cost outcomes; (3) assuming the annual drift in HbA1c was 0.1%, 0.2%, and drift using the UKPDS progression, respectively; (4) assuming treatment switching after 2 years and when HbA1c exceeded 7.0%; (5) costs of complications increasing and decreasing by 10%; (6) applying the UKPDS 68 risk equations to predict the macrovascular complications and mortality; (7)assuming no cardioprotective effect for treatments; and (8) applying BMI disutility value from the Lane et al. [53] study. In addition, probabilistic sensitivity analysis (PSA) was performed.
Compliance with Ethics Guidelines
This article is based on previously conducted clinical trials and does not contain any studies with human participants or animals performed by any of the authors.
Results
Base-Case Analysis
Long-term projection over the 40-year time horizon in patients with T2D uncontrolled on metformin in China revealed that once-weekly semaglutide 0.5 mg and 1.0 mg were associated with an improved discounted life expectancy of 0.08 and 0.12 years, respectively, and improved discounted quality-adjusted life expectancy of 0.16 and 0.22 QALYs, respectively, versus PEG-loxenatide 0.2 mg (Table 2). The clinical benefits were due to the multifactorial risk-reduction effects of semaglutide, resulting in delaying time to onset and reduced cumulative incidence of diabetes-related complications (ESM Table 7).
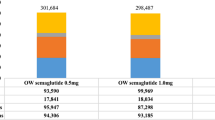
Semaglutide 0.5 mg and 1.0 mg were associated with a cost saving of CNY 19,309 and CNY 10,179 per pa0tient compared with PEG-loxenatide 0.2 mg (see Table 2). The treatment cost, microvascular complications cost, and macrovascular complications cost were all lower for once-weekly semaglutide 0.5 mg than for PEG-loxenatide 0.2 mg. For once-weekly semaglutide 1.0 mg, the treatment cost was slightly higher than that for PEG-loxenatide 0.2 mg, but the increased treatment cost was fully offset by the reduced costs of micro- and macrovascular complications (see Fig. 1).
Sensitivity Analyses
The base-case result was shown to be robust across all one-way sensitivity analyses (Table 3). Once-weekly semaglutide 0.5 mg was dominant in all sensitivity analyses, compared with PEG-loxenatide 0.2 mg. Once-weekly semaglutide 1.0 mg was also dominant compared with PEG-loxenatide 0.2 mg in all but one analysis. When treatment switching occurred at the HbA1c threshold of 7.0%, once-weekly semaglutide 1.0 mg was associated with an ICER value of CNY 7400/QALY gained. In this analysis, patients received once-weekly semaglutide 1.0 mg for 5 years and PEG-loxenatide 0.2 mg for 3 years, resulting in a higher treatment cost for once-weekly semaglutide 1.0 mg.
Using one time gross domestic progduct per capita as the willingness-to-pay threshold, the PSA showed 100% probability that once-weekly semaglutide 0.5 mg and 1.0 mg were both cost-effective compared with PEG-loxenatide 0.2 mg in patients whose diabetes was uncontrolled on metformin (Fig. 2).
Discussion
This study is the first to compare the cost-effectiveness of once-weekly semaglutide with PEG-loxenatide in the Chinese setting. the results showed that, in China, once-weekly semaglutide 0.5 mg and 1.0 mg were both cost-saving treatments compared with PEG-loxenatide in patients with T2D uncontrolled on metformin. Once-weekly semaglutide 0.5 mg and 1.0 mg were associated with improvements of 0.16 and 0.22 QALYs, and with lifetime cost savings of CNY 19,309 and CNY 10,179, respectively, meaning that it dominated PEG-loxenatide.
In this cost-effectiveness analysis, a NMA was performed to obtain the comparative treatment effects of once-weekly semaglutide and PEG-loxenatide in patients with T2D, since there has been no head-to-head clinical trial comparing the efficacy of these treatments. This methodology is aligned with the Chinese Guidelines for Pharmacoeconomic Evaluations, which indicate that clinical data from NMA is preferred in health economic evaluations when there is no clinical data available from the head-to-head trial [34]. Trials included in the NMA were derived from a systematic literature review, and only trials with similar study designs were included to ensure the compatibility of the studies. The NMA showed greater reductions in HbA1c, BMI and SBP and greater improvements in blood lipid levels with once-weekly semaglutide than with PEG-loxenatide. However, further studies based on direct comparison are necessary to reassess the cost-effectiveness of once-weekly semaglutide compared with PEG-loxenatide.
The patients included in this study were assumed to receive once-weekly semaglutide or PEG-loxenatide for 3 years in the base-case analysis before switching to the basal insulin treatment, which is consistent with treatments reported in previous studies [36, 38]. However, in real-world practice, patients may maintain their current treatment if their glucose level is well controlled, which means that treatment with a greater reduction in HbA1c would be associated with a delayed time to insulin intensification. In the sensitivity analysis, when an HbA1c threshold of 7.0% was used as the treatment switching criterion, the groups with once-weekly semaglutide 0.5 mg and 1.0 mg, respectively, were switched to intensification treatment at the fifth year, with a 2-year delay compared to PEG-loxenatide (intensification at the third year). The results showed that once-weekly semaglutide would achieve more quality-adjusted life expectancy compared with the base-case result. Once-weekly semaglutide 0.5 mg was still a cost-saving treatment, while once-weekly semaglutide 1.0 mg was associated with a slight increase in total cost (+ CNY 1964), leading to an ICER value of CNY 7400/QALY gained. Although there is no officially acknowledged willingness-to-pay threshold in China, semaglutide 1.0 mg could still be considered a cost-effective treatment in this scenario.
The treatment of T2D often focuses on lowing HbA1c [54,55,56,57]. However, recent studies have demonstrated further benefits from reductions in other risk factors, including body weight and BMI [58, 59]. The NMA showed that once-weekly semaglutide was associated with greater body weight and BMI reductions versus PEG-loxenatide. In the base-case analysis, the disutility value per unit increase in BMI was set as − 0.006, which is consistent with values reported in other cost-effectiveness studies [60, 61]. However, in a recent study evaluating the relationship between body weight and quality of life in Canadian patients with T2D, researchers observed that for each unit increase in BMI, the utility of the patient would decrease by 0.0472 [53]. In the sensitivity analysis of this study, once-weekly semaglutide showed a greater increase in quality-adjusted life expectancy (+ 0.74 QALYs for semaglutide 0.5 mg, + 0.95 QALYs for semaglutide 1.0 mg) compared with PEG-loxenatide 0.2 mg when the larger disutility value in BMI was applied.
A previous systematic literature review on the cost-effectiveness analysis of once-weekly semaglutide versus other GLP-1RAs in T2D found that the cardiovascular benefit of once-weekly semaglutide may have been under-estimated in previous studies [62]. The importance of HbA1c in long-term cardiovascular risk has been confirmed by a UKPDS study, which showed that well-controlled HbA1c could reduce macrovascular complications after many years [63]. However, increasingly more CVOTs are finding that some novel hypoglycemic agents can obtain cardiovascular benefits in a relatively short follow-up duration. For example, after a median observation time of 2.1 years in the SUSTAIN 6 clinical trial, once-weekly semaglutide was shown to significantly reduce the rate of nonfatal myocardial infarction, nonfatal stroke and cardiovascular death in patients with T2D who were at high cardiovascular risk [9]. Data derived from SUSTAIN 6 were applied in our study to inform the cardiovascular protective effect of once-weekly semaglutide. Since there was no evidence for PEG-loxenatide, it was assumed in this study that PEG-loxenatide has no cardioprotective effects. In addition, Naveed et al. [64] reported that the exendin-4 based GLP-1 RAs may have no significant effect in reducing the incidence of major adverse cardiovascular events. It should be noted that the patient population in the SUSTAIN 6 study consisted of patients with T2D and cardiovascular risk, which was different from the target population in the present study. However, the study by Naveed et al. [64] found that the cardiovascular protective effect of GLP-1 RAs in patients with established cardiovascular disease was not statistically significantly different from that of patients without the established cardiovascular disease. In order to test the impact of the uncertainty of cardioprotective effects on the results, a sensitivity analysis which applied no cardiovascular protective effect to GLP-1 RA treatments was conducted. The results showed that once-weekly semaglutide was still a dominant option compared with PEG-loxenatide.
There are several limitations to the research reported here. First, the treatment effects were derived from treatment-naïve patients; this patient population is slightly different from the patients in the target population who were being adequately controlled on metformin monotherapy. The target population was consistent with the approved indications of once-weekly semaglutide in China. Nevertheless, the clinical trials of PEG-loxenatide were conducted only in treatment-naïve patients with T2D. To ensure the comparability of studies, the study of Sorli et al. [14] and the study of Shuai et al. [25] were included in the NMA to obtain the treatment effects of once-weekly semaglutide and PEG-loxenatide, both of which were performed in treatment-naïve patients. Further analysis based on clinical outcomes of patients with T2D uncontrolled on metformin would be necessary to reassess the cost-effectiveness of once-weekly compared with PEG-loxenatide.
Second, this study was aimed to predict long-term outcomes from relatively short-term clinical trial data, which ware inherent to all long-term health economic analyses. Third, the assumption that the treatment compliance was 100% may not reflect real-world practice. Fourth, some health utility data were from studies in other countries due to the lack of local data, which may not fully reflect the situation of the Chinese population. Future studies addressing these shortcomings with domestic and real-world data are suggested.
Conclusion
The results of this study suggest that compared with PEG-loxenatide 0.2 mg, subcutaneous once-weekly semaglutide 0.5 mg and 1.0 mg are both cost-saving treatments for patients with T2D inadequately controlled on metformin, from a perspective of Chinese healthcare systems.
References
Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369: m997.
Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Diabetes Mellit. 2021;13(04):315–409.
Wang L, Peng W, Zhao Z, et al. Prevalence and treatment of diabetes in China, 2013–2018. JAMA. 2021;326(24):2498–506.
International Diabetes Federation. IDF Diabetes atlas. 2019. https://diabetesatlas.org/atlas/ninth-edition/. Accessed 2 June 2022.
National Bureau of Statistics of China. China statistical yearbook (2020 edition). 2020. http://www.stats.gov.cn/tjsj/ndsj/2020/indexch.htm. Accessed 2 June 2022.
Einarson T, Acs A, Ludwig C, Panton HU. Economic burden of cardiovascular disease in type 2 diabetes: a systematic review. Value Health. 2018;21:881–90.
Shen Y, Li F, Zhu B, et al. Accounting and analysis of curative expenditure on diabetes treatment of Shanghai in 2018. Chin Health Econ. 2022;3(41):56–9.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Gerstein HC, Colhoun HM, Agenais DGR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10196):121–30.
Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29.
Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J Clin Pharmacol. 2015;55(5):497–504.
Lau J, Bloch P, Schaffer L, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58:7370–80.
Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–60.
Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–54.
Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–66.
Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355–66.
Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291–301.
Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–86.
Lingvay I, Catarig AM, Frias JP, et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(11):834–44.
Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356–67.
Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100–9.
Ji L, Dong X, Li Y, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes Metab. 2021;23(2):404–14.
Ruan Z, Ung COL, Shen Y, et al. Long-term cost-effectiveness analysis of once-weekly semaglutide versus dulaglutide in patients with type 2 diabetes with inadequate glycemic control in China. Diabetes Ther. 2022;13(10):1737-53.
Shuai Y, Yang GY, Zhang Q, et al. Efficacy and safety of polyethylene glycol loxenatide monotherapy in type 2 diabetes patients: a multicentre, randomized, double-blind, placebo-controlled phase 3a clinical trial. Diabetes Obes Metab. 2021;23(1):116–24.
Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–59.
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33.
Kiadaliri AA, Gerdtham UG, Nilsson P, Eliasson B, Gudbjörnsdottir S, Carlsson KS. Towards renewed health economic simulation of type 2 diabetes: risk equations for first and second cardiovascular events from Swedish register data. PLoS ONE. 2013;8(5): e62650.
Davis WA, Knuiman MW, Davis TM. An Australian cardiovascular risk equation for type 2 diabetes: the Fremantle Diabetes Study. Intern Med J. 2010;40(4):286–92.
Bagust A, Hopkinson PK, Maier W, Currie CJ. An economic model of the long-term health care burden of Type II diabetes. Diabetologia. 2001;44(12):2140–55.
Brown JB, Russell A, Chan W, Pedula K, Aickin M. The global diabetes model: user friendly version 3.0. Diabetes Res Clin Pract. 2000;50(Suppl 3):S15-46.
Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. I. Model construction and assumptions. Diabetes Care. 1997;20(5):725–34.
Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9.
Liu GG. China guidelines for pharmacoeconomic evaluations. Beijing: China Market Press; 2020.
Wu J, He X, Liu Y. Cost-effectiveness analysis of insulin aspart 30 versus insulin glargine in patients with type 2 diabetes in China. Chin Pharm J. 2016;51(3):242–7.
Malkin SJP, Russel-Szymczyk M, Psota M, Hlavinkova L, Hunt B. The management of type 2 diabetes with once-weekly semaglutide versus dulaglutide: a long-term cost-effectiveness analysis in Slovakia. Adv Ther. 2019;36(8):2034–51.
Hunt B, Malkin SJP, Moes RGJ, Huisman EL, Vandebrouck T, Wolffenbuttel BHR. Once-weekly semaglutide for patients with type 2 diabetes: a cost-effectiveness analysis in the Netherlands. BMJ Open Diabetes Res Care. 2019;7(1): e000705.
Gorgojo-Martínez JJ, Malkin SJP, Martín V, Hallén N, Hunt B. Assessing the cost-effectiveness of a once-weekly GLP-1 analogue versus an SGLT-2 inhibitor in the Spanish setting: Once-weekly semaglutide versus empagliflozin. J Med Econ. 2020;23(2):193–203.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3): e1003583.
Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–43.
Lundqvist A, Steen Carlsson K, Johansen P, Andersson E, Willis M. Validation of the IHE cohort model of type 2 diabetes and the impact of choice of macrovascular risk equations. PLoS ONE. 2014;9(10): e110235.
Cai X, Yang W, Gao X, Zhou L, Han X, Ji L. Meta-analysis of the insulin dosage in Chinese type 2 diabetic patients receiving insulin treatment. Chin J Diabetes. 2016;24(6):490–507.
He X, Zhang Y, Ruan Z, Li L, Wu J. The prevalence and related direct medical costs of chronic complications among patients with type 2 diabetes in China. Chin J Endocrinol Metab. 2019;3(35):200–5.
Xie X, Vondeling H. Cost-utility analysis of intensive blood glucose control with metformin versus usual care in overweight type 2 diabetes mellitus patients in Beijing PR China. Value Health. 2008;11(Suppl 1):S23-32.
Su W, Li C, Zhang L, Lin Z, Tan J, Xuan J. Meta-analysis and cost-effectiveness analysis of insulin glargine 100 U/mL versus insulin degludec for the treatment of type 2 diabetes in China. Diabetes Ther. 2019;10(5):1969–84.
Duan X, Li Y, Liu Q, Liu L, Li C. Epidemiological characteristics, medical costs and healthcare resource utilization of diabetes-related complications among Chinese patients with type 2 diabetes mellitus. Expert Rev Pharmacoecon Outcomes Res. 2020;20(5):513–21.
Deng J, Gu S, Shao H, Dong H, Zou D, Shi L. Cost-effectiveness analysis of exenatide twice daily (BID) vs insulin glargine once daily (QD) as add-on therapy in Chinese patients with type 2 diabetes mellitus inadequately controlled by oral therapies. J Med Econ. 2015;18(11):974–89.
Men P, Qu S, Luo W, Li C, Zhai S. Comparison of lixisenatide in combination with basal insulin vs other insulin regimens for the treatment of patients with type 2 diabetes inadequately controlled by basal insulin: systematic review, network meta-analysis and cost-effectiveness analysis. Diabetes Obes Metab. 2020;22(1):107–15.
Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–70.
Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002;22(4):340–9.
Pan CW, Sun HP, Zhou HJ, et al. Valuing health-related quality of life in type 2 diabetes patients in China. Med Decis Making. 2016;36(2):234–41.
Johansen P, Håkan-Bloch J, Liu AR, Bech PG, Persson S, Leiter LA. Cost effectiveness of once-weekly semaglutide versus once-weekly dulaglutide in the treatment of type 2 diabetes in Canada. Pharmacoecon Open. 2019;3(4):537–50.
Lane S, Levy AR, Mukherjee J, Sambrook J, Tildesley H. The impact on utilities of differences in body weight among Canadian patients with type 2 diabetes. Curr Med Res Opin. 2014;30(7):1267–73.
Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–30.
Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152(1):27–38.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1988;352(9131):837–53.
Wilding JP. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68(6):682–91.
Fruh SM. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29(S1):S3-s14.
Gæde P, Johansen P, Tikkanen CK, Pollock RF, Hunt B, Malkin SJP. Management of patients with type 2 diabetes with once-weekly semaglutide versus dulaglutide, exenatide ER, liraglutide and lixisenatide: a cost-effectiveness analysis in the Danish ssetting. Diabetes Ther. 2019;10(4):1297–317.
Viljoen A, Hoxer CS, Johansen P, Malkin S, Hunt B, Bain SC. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21(3):611–21.
Ruan Z, Yang L, Shi H, et al. The cost-effectiveness of once-weekly semaglutide compared with other GLP-1 receptor agonists in type 2 Diabetes: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2021;21(2):221–33.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Sattar N, Lee MMY, Kristensen SL et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–62.
Acknowledgements
The authors would like to thank for the comments on early visions from their colleagues at the University of Macau.
Funding
Funding for this study and the journal’s Rapid Service Fees charges was provided by Novo Nordisk (China) Pharmaceuticals Co., Ltd. This supplement has been sponsored by Novo Nordisk (China) Pharmaceuticals Co., Ltd..
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. The study was conceived and designed by Hao Hu and Lixin Guo. Zhen Ruan and Yang Shen conducted the modeling analysis. Lei Liu and Yawen Zhang drafted the manuscript. The final vision was reviewed, revised, and approved by all authors.
Disclosures
Yawen Zhang is an employee of Novo Nordisk. Other authors declare no conflict of interest.
Prior Presentation
The preliminary result of manuscript is based on work that is presented at ISPOR Europe 2022, 6-9 November, Vienna, Austria and virtually.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article and supplementary material. Access to the IHE-DCM is at the discretion of the Swedish IHE, Lund, Sweden, which holds the proprietary right.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, L., Ruan, Z., Ung, C.O.L. et al. Long-Term Cost-Effectiveness of Subcutaneous Once-Weekly Semaglutide Versus Polyethylene Glycol Loxenatide for Treatment of Type 2 Diabetes Mellitus in China. Diabetes Ther 14, 93–107 (2023). https://doi.org/10.1007/s13300-022-01336-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01336-7