Abstract
We present a case of prostate cancer (PC) developing in a hypogonadal patient with well-controlled type 1 diabetes. The purpose of reporting this case is to emphasize that regular prostate examinations and prostate-specific antigen (PSA) measurements should be preformed in the diabetic male, even though the incidence of PC is lower in this group of patients. In addition, these examinations and tests need to be preformed even in the hypogonadal patient with diabetes since the presence of a low serum testosterone (T) level does not preclude the development of PC. This is because the development of PC is not related to serum androgen levels but to the androgen levels within the prostate, and dihydrotestosterone (DHT) levels and not T levels within the prostate gland are responsible for the development of PC. In the hypogonadal male, intraprostatic DHT may be high since DHT can be formed from adrenal androgens, particularly androstenedione, through activation of 5α-reductase 2, which is the minority enzyme in the normal prostate but becomes the major enzyme in the formation and growth of PC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Low testosterone is not protective against prostate cancer. |
DHT production is increased in the prostate and testicle even testosterone is present. |
Diabetes is associated with a lower risk of adenocarcinoma of the prostate. |
Intraprostate levels of androgens and not serum levels are associated with prostate cancer. |
Key androgen in prostate cancer is dihydrosterone (DHT). |
This paper emphasizes the need for annual prostate exam and PSA in both diabetic and hypogonadal patients. |
Introduction
Diabetes is associated with increased incidences of cancers of the pancreas, liver, biliary tract, endometrium, kidney, colon, and esophagus [1]. However, surprisingly diabetes is associated with a lower risk of adenocarcinoma of the prostate (PC). In the Health Professionals Follow-up study the risk of PC was reduced by 7% (95% CI 0.74–0.94) in those with diabetes. Obesity and longer duration of diabetes were also found to be particularly protective against the development of PC [2]. While diabetes was not classified in this study we can assume that the risk was likely higher with type 1 diabetes since a higher HbA1c has been associated with more aggressive PCs, an increased number of metastases (particularly castration-resistant metastases) as well as a higher rate of extraprostatic extension and a higher Gleason score [3,4,5].
While the increased risk of more advanced disease in the subject with diabetes is thought to be due to an increased expression of metalloproteinases and chemokine ligands, the decreased overall incidence of PC with diabetes has been hypothesized to be due to decreased detection of PC, the protective effect of diabetic medications, and diabetes-induced vascular damage to the prostate [6]. However, the most hypothesized explanation for the decrease in PC with diabetes are decreases in sex hormone levels and growth factors [6]. Furthermore, prostate-specific antigen (PSA) levels have been shown to be 21.6% lower in men with diabetes compared with men without diabetes, and further decreased with the number of years post diagnosis of PC which again is postulated to be due to lower androgen levels [7, 8]. However, low androgen levels accounting for the decreased risk of PC in the patient with diabetes is simply a hypothesis and there is absolutely no evidence to indicate that testosterone or any other measurable sex hormone plays a role in the development of PC in the patient with diabetes. Furthermore, the development of PC is widely perceived to be accelerated in the presence of elevated testosterone levels probably because regular measurement of PSA levels is medicolegally mandated in patients on testosterone replacement therapy.
The myth of androgen-induced PC is further propagated by the belief that castration is “a cure” for advanced and/or metastatic PC. However, the effect of castration is at best temporary [9]. A study of 6933 PC cases after 6.8 years showed that those in the lowest decile of free testosterone levels had a lower overall incidence of PC than those in the second to tenth decile but those in the first decile also had a non-significantly higher risk (OR 1.56, 95% CI 0.95–2.57) of high grade PC [10]. In fact, on the basis of recently available safety data, therapy with testosterone has actually been recommended in those with PC in whom therapy has been completed [11].
In this case report we present a patient with chronic testosterone deficiency and type 1 diabetes who did not tolerate testosterone replacement and thus was only given this for a few months. Four years later the patient had a prostate nodule biopsied and was found to have adenocarcinoma of the prostate. Many would assume this patient was at low risk because of being ideal body weight, having well-controlled type 1 diabetes, and having testosterone deficiency. This report emphasizes that testosterone deficiency affords no protection from prostate cancer and should play no role in the discussion between doctors and patients regarding PSA testing and prostate examinations. It should also be noted that these individuals are at increased risk of having high grade PC.
Case Report
A 42-year-old man, married with four children, who had just been diagnosed with type 1 diabetes (GAD positive 26.7, C-peptide 2.5, HbA1c 6.1%) was found to have a low serum testosterone level of 170 ng/dl (normal range 260–923 ng/dl). Follicular stimulating hormone, luteinizing hormone, prolactin, and estradiol levels were normal. His only symptoms of hypogonadism were fatigue and perhaps a low libido. On examination testicles were of normal size as was the non-nodular prostate gland. There was no muscle wasting and distribution of body hair was normal. He shaved on a daily basis and had bilateral temporal recession of the hairline as well as mild balding in the crown area.
He was diagnosed as having idiopathic male hypogonadism and started on intramuscular testosterone enanthate 200 mg every 2 weeks. On this regimen he had an excessive increase in libido and he felt that his skin was “greasy”. Because of these complaints and the development of polycythemia (Hb 18.0 g/dl) his treatment was converted to a transdermal testosterone cream which he also did not like because of its “stickiness”. He therefore discontinued testosterone replacement therapy and as a result his serum testosterone dropped back from 241 ng/ml to 123 ng/ml. In spite of this drop in serum testosterone he did not develop symptoms that could be related to hypogonadism.
Four years later at age 48 on a routine physical examination he was found to have a nodule on his prostate which was accompanied by a PSA level of 3.5 ng/ml (normal less than 4). Three months later the nodule disappeared without a change in the PSA level. Two years later at age 50 the prostate nodule was found to have recured and his PSA level rose to 4.2 ng/ml. On prostate biopsy he was found to have an adenocarcinoma of the prostate (Gleason score 7, stage T2a) for which he had successful brachytherapy. Prior to therapy his 5α-dihydrosterone level was 170 pg/ml (normal range 108–709 pg/ml) and following brachytherapy his PSA dropped to 0.35 mg/dl. Consent to publish was obtained from the patient by Dr. Bell.
Discussion
We have presented a patient with excellently controlled type 1 diabetes who was also testosterone deficient and who developed PC. At the time of diagnosis he had been off testosterone replacement therapy for 4 years.
Perhaps, on the basis of the utilization of castration therapy for PC, it is widely and incorrectly believed that testosterone therapy increases the risk of PC. This is not the case since castration is at best only a temporary solution for PC.
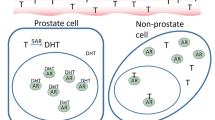
Furthermore, the androgen levels that determine the development of PC are the levels that are present within the prostate gland and not the serum levels. In addition, there is no correlation between the levels of androgens within the prostate gland and the serum levels. An example of this is in Kleinfelter syndrome where the levels of intratesticular androgens are actually increased in spite of very low serum levels [12].
Man is unique among animal species in that there is a high secretion rate of adrenal steroids, particularly androstenedione, that can be converted into more potent androgens in both the testicles and the prostate. The androgen that is dominant in the prostate is dihydrotestosterone (DHT), the level of which is unrelated to either the serum levels of either DHT or testosterone, and DHT is the hormone within the prostate that controls the development and growth of PC [13, 14].
DHT is formed from testosterone by the activity of 5α-reductase 1, which in normal prostate tissue is present in lower quantities than that of the more potent 5α-reductase 2. However, in PC this ratio is reversed which results in much higher DHT levels within the prostate [15].
For medicolegal reasons when testosterone replacement therapy is being administered we are encouraged to frequently measure PSA levels. However, with testosterone replacement therapy an increased risk of developing PC has never been documented [16]. Indeed, with completion of the therapy of PC, hypogonadism can be safely treated with exogenous testosterone and is currently recommended [11, 17].
Since castration has been utilized to treat metastatic PC it has also been assumed that androgen levels drop to almost zero following castration. However, castration only lowers the levels of DHT by 20% to 30% while testosterone levels are lowered by 94% [18].
Conclusion
In summary, we present a case of a patient with PC who was not protected by either hypogonadism or well-controlled type 1 diabetes. This case emphasizes that testosterone deficiency does not obviate the need for PSA sampling or prostate exams in male patients including patients with diabetes between the ages of 55 and 70 who have had a discussion about risks and benefits of these procedures with their physicians.
References
Strickler HD, Wylie-Rosett J, Rohan T, et al. The relation of type 2 diabetes and cancer. Diabetes Technol Ther. 2001;3(2):263–74.
Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009;124(6):1398–403.
Kim HS, Presti JC Jr, Aronson WJ, et al. Glycemic control and prostate cancer progression: results from the SEARCH database. Prostate. 2010;70(14):1540–6.
Nik-Ahd F, Howard LE, Eisenberg AT, et al. Poorly controlled diabetes increases the risk of metastases and castration-resistant prostate cancer in men undergoing radical prostatectomy: results from the SEARCH database. Cancer. 2019;125(16):2861–7.
Hong SK, Lee ST, Kim SS, et al. Significance of preoperative HbA1c level in patients with diabetes mellitus and clinically localized prostate cancer. Prostate. 2009;69(8):820–6.
Pierce BL. Why are diabetics at reduced risk for prostate cancer? A review of the epidemiologic evidence. Urol Oncol. 2012;30(5):735–43.
Werny DM, Saraiya M, Gregg EW. Prostate-specific antigen values in diabetic and nondiabetic US men, 2001–2002. Am J Epidemiol. 2006;164(10):978–83.
Klotz L. Testosterone therapy and prostate cancer–safety concerns are well founded. Nat Rev Urol. 2015;12(1):48–54. https://doi.org/10.1038/nrurol.2014.338.
Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180–92.
Watts EL, Appleby PN, Perez-Cornago A, et al. Low free testosterone and prostate cancer risk: a collaborative analysis of 20 prospective studies. Eur Urol. 2018;74(5):585–94.
Lenfant L, Leon P, Cancel-Tassin G, et al. Testosterone replacement therapy (TRT) and prostate cancer: an updated systematic review with a focus on previous or active localized prostate cancer. Urol Oncol. 2020;38(8):661–70.
Tuttleman K. Intratesticular testosterone is increased in men with Kleinfelter’s syndrome and may not be released into the bloodstream owing to altered testicular vascularization- a preliminary report. Andrology. 2014;2(2):275–81.
Arai S, Shibata Y, Nakamura Y, et al. Development of prostate cancer in a patient with primary hypogonadism: intratumoural steroidogenesis in prostate cancer tissues. Andrology. 2013;1(1):169–74.
Petrow V. The dihydrotestosterone (DHT) hypothesis of prostate cancer and its therapeutic implications. Prostate. 1986;9(4):343–61.
Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108(33):13728–33.
Santella C, Renoux C, Yin H, Yu OHY, Azoulay L. Testosterone replacement therapy and the risk of prostate cancer in men with late-onset hypogonadism. Am J Epidemiol. 2019;188(9):1666–73.
Rodriguez KM, Pastuszak AW, Khera M. The role of testosterone therapy in the setting of prostate cancer. Curr Urol Rep. 2018;19(8):67.
Page ST, Lin DW, Mostaghel EA, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91(10):3850–6.
Acknowledgements
Funding
No funding or sponsorship was received for this study or for publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for the work as a whole.
Author contributions
Authors contributed equally to the manuscript in all areas.
Disclosures
Terri Jerkins and David S. H. Bell have no disclosures to declare.
Compliance with Ethics Guidelines
Consent to publish was obtained from the patient by Dr. Bell.
Data Availability
Data is available upon request from Dr. Bell.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bell, D.S.H., Jerkins, T. Testosterone Deficiency is Not Protective Against the Development of Adenocarcinoma of the Prostate in a Type 1 Diabetic Patient. Diabetes Ther 13, 1115–1119 (2022). https://doi.org/10.1007/s13300-022-01256-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01256-6