Abstract
Introduction
Measurement of glucose levels is the mainstay method of ensuring good glycemic control and preventing complications associated with uncontrolled diabetes. Continuous glucose monitoring enables easy and effective monitoring of interstitial glucose around the clock and hence improves glycemic control.
Objectives
This study aimed to measure the effect of continuous glucose monitoring on glycated hemoglobin (HbA1c) at 3, 6, and 9 months following sensor insertion.
Methods
A retrospective cohort study of pediatric and adolescent type 1 diabetes mellitus patients randomly sampled from 32 Ministry of Health diabetes centers across Saudi Arabia was performed. Patients were subjected to flash glucose monitoring using the FreeStyle® Libre flash glucose monitoring system (Abbott Diabetes Care, Witney, UK), an intermittently scanned continuous glucose monitoring device approved by the Conformité Européenne in 2014. These patients were first-time users of any kind of continuous glucose monitoring system, aged 4–18 years, and received insulin via multiple dose injection or continuous subcutaneous insulin infusion for at least 6 months prior to study start. Patients were excluded if they had used flash glucose monitoring or other interstitial glucose monitoring systems in the past 3 months, were pregnant, or had existing hemoglobinopathies. The flash glucose monitoring sensor was attached to the back of the upper arm at the baseline visit. HbA1c (%) was measured at baseline and 3, 6, and 9 months. Patient demographics were collected from electronic health records.
Results
1,307 patients were included, with a mean age of 11.1 years (standard deviation 3.6 years). Where specified, 51.4% were female. Mean HbA1c significantly reduced from baseline (10.8%) to 3 months (9.8%, p < 0.001), 6 months (9.2%, p < 0.001), and 9 months (9.1%, p < 0.001). For individuals with baseline HbA1c > 9%, mean HbA1c was significantly reduced from baseline (11.7%) to 3 months (10.3%, p < 0.001), 6 months (9.6%, p < 0.001), and 9 months (9.5%, p < 0.001).
Conclusions
Flash glucose monitoring significantly reduced HbA1c levels at 3, 6, and 9 months following sensor insertion. This reduction was greatest in those patients with higher HbA1c at baseline (> 9%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Measurement of glucose levels is the mainstay method of ensuring good glycemic control and preventing complications associated with uncontrolled diabetes. |
This study aimed to measure the effect of continuous glucose monitoring on glycated hemoglobin (HbA1c) in a cohort of 1,307 pediatric and adolescent type 1 diabetes mellitus patients randomly sampled from 32 Ministry of Health diabetes centers across Saudi Arabia. |
This is the first audit of continuous glucose monitoring technology in the Middle East region, and this study will serve to guide practice and the adoption of technology in clinical pathways. |
What was learned from the study? |
Mean HbA1c significantly reduced from baseline (10.8%) to 3 months (9.8%, p < 0.001), 6 months (9.2%, p < 0.001), and 9 months (9.1%, p < 0.001). |
For individuals with baseline HbA1c > 9%, mean HbA1c was significantly reduced from baseline (11.7%) to 3 months (10.3%, p < 0.001), 6 months (9.6%, p < 0.001), and 9 months (9.5%, p < 0.001) |
Flash glucose monitoring significantly reduced HbA1c levels at 3, 6, and 9 months following sensor insertion, and this reduction was greatest in those patients with higher HbA1c at baseline (> 9%). |
Introduction
Diabetes mellitus is a chronic metabolic disease affecting 8.8% of the global population aged between 20 and 79 years [1]. Its prevalence is rising fast, with a predicted prevalence of 10.4% of the global population aged between 20 and 79 years by 2040 [1]. Type 1 diabetes mellitus (T1DM) is characterized by a lack of insulin production by the pancreas. The incidence of T1DM in the Kingdom of Saudi Arabia has increased drastically in the last three decades, with increased rates in young infants [2]. The International Diabetes Federation (IDF) Diabetes Atlas, 9th edition, reported that 27,784 children and adolescents in Saudi Arabia suffered from T1DM, with 3700 new cases diagnosed each year [3, 4].
Measurement of glycated hemoglobin (HbA1c) enables the assessment of glycemic control in diabetic patients [5]. Ensuring good glycemic control is essential to prevent complications associated with uncontrolled diabetes [5, 6]. Microvascular complications and long-term macrovascular disease can be minimized via intensive therapy, as well as the measurement and reduction of HbA1c [7,8,9].
Continuous glucose monitoring (CGM) enables blood glucose levels to be measured continuously, providing a much more convenient solution for glucose monitoring than self-monitoring of blood glucose (SMBG) using fingerpricks [10]. The flash glucose monitoring system is an intermittently scanned CGM device approved by the Conformité Européenne in 2014 and the US Food and Drug Administration in 2017 [11]. Flash glucose monitoring measures the glucose levels in the interstitial fluid from cells underneath the skin [12]. The device has been shown to effectively measure glucose levels in adult [6] and pediatric populations [13]. Similar to other CGM devices, flash glucose monitoring has been shown to measure time spent in range, time in hypoglycemia, time in hyperglycemia, and glucose variability, with the added advantage of being factory calibrated [12, 14]. The device is accurate, safe, and highly accepted by users or their caregivers [15]. Furthermore, flash glucose monitoring has been shown to reduce HbA1c levels and hypoglycemia events as well as to improve quality of life (diabetes distress and sleep quality) in type 1 diabetes patients [16, 17].
Several randomized clinical trials have demonstrated that CGM leads to reductions in HbA1c, hypoglycemia, and glycemic variability compared with SMBG testing [18,19,20,21,22]. A recent meta-analysis investigating the impact of flash glucose monitoring on glycemic control demonstrated a mean change in HbA1c at 2–4 months across 29 studies of − 0.55% (95% CI − 0.70, − 0.39) following the use of flash glucose monitoring [23]. Flash glucose monitoring has been shown to lead to significant and sustained reductions in HbA1c in both adults and children with T1DM, as well as adults with type 2 diabetes mellitus [23].
The aim of this study was to evaluate the effect of flash glucose monitoring on HbA1c levels at 3, 6, and 9 months following sensor insertion in a population of pediatric and adolescent T1DM patients across Saudi Arabia.
Methods
Study Design
A retrospective cohort study was conducted in pediatric and adolescent patients with T1DM from 32 Ministry of Health (MOH) diabetes centers and units across the Kingdom of Saudi Arabia between September 2018 and October 2019. Patients were randomly sampled, with an average of 50 patients who met the inclusion criteria being randomly sampled from each of the diabetes centers. Patients were included if they were first-time users of flash glucose monitoring, aged 4–18 years, received insulin via multiple dose injection or continuous subcutaneous insulin infusion for at least 6 months prior to study start, and were eligible to use flash glucose monitoring as per the current Saudi MOH criteria. Patients were excluded if they had used flash glucose monitoring or other interstitial glucose monitoring systems in the past 3 months, were pregnant, or had existing hemoglobinopathies.
Patients were assessed at a baseline visit and followed up at 3, 6, and 9 months. At the baseline visit, the flash glucose monitoring sensor was attached to the back of the upper arm by a member of the diabetes team at each MOH diabetes center.
Variables
HbA1c (%) was measured at baseline and 3, 6, and 9 months using a local laboratory test. Patient demographics (age and gender) were collected from electronic health records.
Analysis
Descriptive statistics were used, including measures of central tendency and dispersion for continuous variables (mean, standard deviation [SD], median, and interquartile range [IQR]) and frequencies with proportions for categorical variables. The distribution of the data was assessed for normality using the Q–Q plot and the Shapiro–Wilk test. The paired t-test was used to assess the significance of differences between baseline HbA1c and the 3-, 6-, and 9-month HbA1c values. Stratified analysis was performed by baseline HbA1c category (≤ 7%, 7–9%, > 9%). Missing data are presented as such, without imputation. All statistical analyses were performed using StataIC (StataCorp, version 16). Mapping of the 32 MOH diabetes centers was performed using QGIS (version 3.60 ‘Hannover’).
Ethics
This was a retrospective cohort analysis audit performed as part of Kingdom of Saudi Arabia Ministry of Health practice guidelines. Ethics committee approval was sought and obtained from King Fahad Medical City. All data were retrieved from MOH clinics and de-identified.
Results
Diabetes Centers
The geographic distribution of the 32 diabetes centers across Saudi Arabia is shown in Fig. 1.
Cohort Demographics
A total of 1568 patients met the inclusion criteria for the study. 261 patients were lost to follow-up or had incomplete files. Analysis was performed on the remaining 1,307 patients. The mean age of the patients was 11.1 years (SD 3.6 years). Where specified, females comprised 51.4% of the patients and males 48.6%. Mean HbA1c at baseline was 10.8% (SD 2.2%). Baseline characteristics are summarized in Table 1.
HbA1c glycated hemoglobin, IQR interquartile range, SD standard deviation
Changes in HbA1c from Baseline
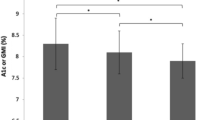
Compared with baseline HbA1c, mean HbA1c was significantly reduced at 3 months (9.8% [SD 1.9], p < 0.001), 6 months (9.2% [SD 1.7], p < 0.001), and 9 months (9.1% [SD 1.9], p < 0.001) (Fig. 2). This reduction was most significant in patients with HbA1c > 9% at baseline (Fig. 3).
Discussion
The results of this study demonstrate significant reductions in HbA1c levels at 3, 6, and 9 months following flash glucose monitoring sensor insertion. Such a trend has been observed in previous studies, with a recent meta-analysis reporting a mean change of − 0.55% (95% CI − 0.70, − 0.39) in HbA1c at 2–4 months following the use of flash glucose monitoring across 29 studies, demonstrating significant and sustained decreases in HbA1c in adults with type 1 and type 2 diabetes as well as children with type 1 diabetes [23]. The meta-analysis included data from randomized control trials and observational studies that included between 6 and 278 diabetes patients, totaling 1,470 patients from heterogeneous study populations [23]. In our study, we present a large dataset of 1,307 patients, providing strong evidence for improved glycemic control in this set of diabetic patients. In addition, several randomized control trials have demonstrated that CGM leads to reductions in HbA1c, hypoglycemia, and glycemic variability compared with SMBG testing [18,19,20,21,22].
The mean HbA1c of this population of patients is high, at 10.8%. This is similar to another study, which described a population of 510 children and adolescents with T1DM under the age of 18 years at King Fahad Medical City, Riyadh [24]. This study reported a mean HbA1c of 10.6%. Such high HbA1c levels reflect poor diabetes control within children and adolescents with T1DM in Saudi Arabia and highlight the importance of effective diabetes management and patient education.
As with all cohort studies, there are limitations to this study. Selection bias resulting from the inclusion of patients from MOH diabetes centers only is a possibility. Nevertheless, the centers are well distributed across Saudi Arabia and are likely representative of the diabetes centers across the country. Furthermore, loss to follow-up bias (due to certain types of patients being lost to follow-up from the study) is also a possibility. The lack of information relating to glucose metrics such as time in range (euglycemia), time above range (hyperglycemia), or time below range (hypoglycemia) as well as glycemic variability and diabetic ketoacidosis events is a further limitation to the study. At the time of the study, diabetes centers did not use Libreview to collect these glucose metrics. Furthermore, additional clinical information is also lacking, such as time since diagnosis, body mass index, and insulin regimen. This is a result of the lack of availability of such data within the electronic health records. Future investigations will include the analysis of longer-term effects of flash glucose monitoring on HbA1c, as well as the analysis of glucose metrics such as time in euglycemia, hyperglycemia, and hypoglycemia, diabetic ketoacidosis, and glycemic variability. Such investigations will further reinforce the benefits of flash glucose monitoring in the diabetic population in Saudi Arabia.
Conclusions
Flash glucose monitoring significantly reduced HbA1c levels at 3, 6, and 9 months following sensor insertion. This reduction was greatest in patients with higher HbA1c at baseline (> 9%).
References
Herman WH. The global burden of diabetes: an overview. In: Dagogo-Jack S, editor. Diabetes mellitus in developing countries and underserved communities. Berlin: Springer International Publishing; 2017.
Robert AA, Al-Dawish A, Mujammami M, Dawish MAA. Type 1 diabetes mellitus in Saudi Arabia: a soaring epidemic. Int J Pediatr. 2018;2018:9408370.
International Diabetes Federation. Saudi Arabia Country report 2010—2045, 9th edition. 2019 [updated 25 November 2019]. Available from: https://diabetesatlas.org/data/en/country/174/sa.html.
International Diabetes Federation. IDF Diabetes Atlas: Middle East and North Africa, 9th edition. 2019 [updated 25 November 2019]. Available from: https://diabetesatlas.org/data/en/region/4/mena.html.
Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther. 2017;19(S2):S4–11.
Olafsdottir AF, Attvall S, Sandgren U, Dahlqvist S, Pivodic A, Skrtic S, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor FreeStyle Libre in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):164–72.
Diabetes C, Complications Trial Research G, Nathan DM, Genuth S, Lachin J, Cleary P, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
Adolfsson P, Rentoul D, Klinkenbijl B, Parkin CG. Hypoglycaemia remains the key obstacle to optimal glycaemic control—continuous glucose monitoring is the solution. Eur Endocrinol. 2018;14(2):50–6.
FDA. FDA approves first continuous glucose monitoring system for adults not requiring blood sample calibration. 2017. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-continuous-glucose-monitoring-system-adults-not-requiring-blood-sample.
Al Hayek AA, Robert AA, Al Dawish MA. Evaluation of FreeStyle Libre flash glucose monitoring system on glycemic control, health-related quality of life, and fear of hypoglycemia in patients with type 1 diabetes. Clin Med Insights Endocrinol Diabetes. 2017;10:1179551417746957.
Massa GG, Gys I, Op ‘t Eyndt A, Bevilacqua E, Wijnands A, Declercq P, et al. Evaluation of the FreeStyle(R) Libre flash glucose monitoring system in children and adolescents with type 1 diabetes. Horm Res Paediatr. 2018;89(3):189–99.
Bonora B, Maran A, Ciciliot S, Avogaro A, Fadini GP. Head-to-head comparison between flash and continuous glucose monitoring systems in outpatients with type 1 diabetes. J Endocrinol Invest. 2016;39(12):1391–9.
Edge J, Acerini C, Campbell F, Hamilton-Shield J, Moudiotis C, Rahman S, et al. An alternative sensor-based method for glucose monitoring in children and young people with diabetes. Arch Dis Child. 2017;102(6):543–9.
Dover AR, Stimson RH, Zammitt NN, Gibb FW. Flash glucose monitoring improves outcomes in a type 1 diabetes clinic. J Diabetes Sci Technol. 2017;11(2):442–3.
Al Hayek AA, Al Dawish MA. Assessing diabetes distress and sleep quality in young adults with type 1 diabetes using FreeStyle Libre: a prospective cohort study. Diabetes Ther. 2020;11(7):1551–62.
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76.
Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–74.
Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371–8.
Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379–87.
Battelino T, Conget I, Olsen B, Schutz-Fuhrmann I, Hommel E, Hoogma R, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155–62.
Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther. 2020;11(1):83–95.
Alsaheel AY, Alayed SI, Alotaibi YM, Alfahhad AA, Alothman OM, Alnefaie HF. Mean glycosylated hemoglobin in children with type 1 diabetes at King Fahad Medical City, Riyadh, Saudi Arabia. J Fam Community Med. 2020;27(3):163–7.
Acknowledgements
Funding
The authors received no funding for this study. The journal Rapid Service Fee was funded by Abbott Diabetes Care.
Medical Writing Assistance
Statistical analysis and medical writing support were provided by Dr. Abigail Holland of Connect Communications, Dubai, and funded by Abbott Diabetes Care.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the conception and design of the study, acquisition of the data, data analysis, drafting and review of the manuscript as well as approval of the final manuscript. All authors agree to be accountable for all aspects of the work.
Disclosures
The authors declare no conflicts of interest.
Compliance with Ethics Guidelines
Ethics committee approval was sought and obtained from King Fahad Medical City. All data retrieved from clinics as part of the audit was de-identified and no informed consent was required.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alharbi, M.Y., Albunyan, A., Al Nahari, A. et al. Measuring the Impact of Flash Glucose Monitoring in a Pediatric Population in Saudi Arabia: A Retrospective Cohort Study. Diabetes Ther 13, 1139–1146 (2022). https://doi.org/10.1007/s13300-022-01224-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01224-0