Abstract
Aim
To compare the real-world effectiveness of once-weekly dulaglutide 1.5 mg with insulin in injectable-naïve patients with type 2 diabetes mellitus (T2DM).
Methods
A non-interventional, non-randomised, observational, single-site retrospective chart review enrolled 150 patients, 75 receiving dulaglutide or insulin.
Data were collected from electronic medical records of patients with T2DM who were initiated on insulin between October 2010 and May 2017, and patients initiated on dulaglutide between May 2018 and October 2019. A doubly robust approach was used to adjust for potential selection bias with augmented inverse probability weights used to estimate the average treatment effect.
Results
HbA1c favoured dulaglutide with an average change of − 1.6% vs − 0.8% for insulin, with an average treatment effect difference of 0.8% (95% confidence interval (CI) 0.4–1.2%) at 3 months. At 6 months, 58.7% of the dulaglutide group reached a target HbA1c of ≤ 7% compared with 20.0% of the insulin group: average treatment effect difference of 21.3% (95% CI 2.7–43.1). The dulaglutide group lost 2.4 kg compared to the insulin group which gained 2.0 kg: average difference of 4.4 kg (95% CI 2.6–7.3) at 6 months. The incidence of hypoglycaemic events was 12 (16.0%) occurrences in the dulaglutide group compared to 33 (44.0%) in the insulin group.
Conclusion
Once-weekly dulaglutide demonstrated greater HbA1c reduction, weight loss and reduced hypoglycaemia compared to insulin, in a real-world practice setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is limited real-world evidence for treatment benefits of dulaglutide in type 2 diabetes mellitus. |
This study aimed to compare the real-world effectiveness of dulaglutide compared with insulin in injectable-naïve patients with type 2 diabetes mellitus. |
What was learned from the study? |
Dulaglutide showed a greater HbA1c reduction compared to insulin over 6 months despite lower baseline HbA1c. Dulaglutide also showed greater weight loss and a lower incidence of hypoglycaemia compared to insulin. |
The beneficial effects and therapeutic benefits of dulaglutide therapy previously observed in randomised control cohorts can be translated into the real-world setting. |
Introduction
Type 2 diabetes mellitus (T2DM) is often treated with a combination of dietary modification and anti-hyperglycaemic (AHG) therapy. After metformin, additional AHG agents may need to be introduced in individuals with persisting hyperglycaemia [1]. The American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) released their first position consensus statement on this topic in 2012 [2], recommending that, after metformin, one of several additional AHG drug classes could be considered with the precise choice left to the clinician and to be individualized for each patient.
In 2018, the ADA-EASD for the first time then recommended guidance in the management of T2DM based on the presence of cardiovascular and/or renal disease; this was a major departure from previous position statements where there was no guidance based on these comorbidities [3]. Most recently, the current position statement recommends that for patients with established atherosclerotic cardiovascular disease (ASCVD) or indicators of high ASCVD risk, heart failure, or chronic kidney disease (CKD), a sodium-glucose co-transporter 2 (SGLT2) inhibitor or glucagon-like peptide 1 receptor agonist (GLP-1 RA) with demonstrated cardiovascular disease (CVD) benefit be considered whilst taking into account patient-specific factors, independent of HbA1c or metformin use [4].
The ADA-EASD joint consensus in 2018 also recommended for the first time that GLP-1 RAs should be the preferred choice of injectable therapy, rather than insulin, when glycated haemoglobin (HbA1c) levels are above target despite maximally tolerated oral AHG therapy, irrespective of heart or kidney disease. This is barring contraindications and not in cases of extreme and symptomatic hyperglycaemia in which case insulin would be recommended [3]. This recommendation is based on several trials showing equivalent glucose-lowering efficacy of GLP-1 RAs compared with insulin, with less hypoglycaemia and weight gain [5].
GLP-1 RAs modulate glucose homeostasis rather than exogenously replacing insulin [6]. Compared with insulin therapy, they can be dosed in less frequent treatment intervals (weekly GLP-1 RAs) while decreasing hypoglycaemia and providing potential weight loss benefits. Insulin treatment requires potentially more frequent dose titration, intense education, and good patient compliance with intensification of insulin therapy requiring up to four injections per day in some patients.
GLP-1 RAs lead to therapeutic reductions in HbA1c levels, a decreased risk of hypoglycaemia and weight loss [3, 7]. Furthermore, several recent cardiovascular protective randomised control trials have demonstrated a therapeutic advantage of GLP-1 RAs in reducing CVD end points [8–10]. The clear cardiovascular benefits of specific AHG agents within the GLP-1 RA class have led to the new set of recommendations that have addressed this evidence [4]. CVD is the leading cause of death in patients with T2DM, making CVD-protective therapies the preferred agents for the treatment of T2DM [3].
Whilst long-acting GLP-1 RAs have demonstrated good glucose-lowering outcomes, with reduced risk of hypoglycaemia and the promotion of weight loss in clinical trials compared to insulin therapy, some clinicians are still reluctant to prescribe GLP-1 RAs over insulin when injectable therapy is needed [5]. There is limited real-world evidence relating to whether the treatment benefits observed for dulaglutide in randomised controlled trials (RCTs) translate into individuals receiving the drug in routine clinical practice. This is because common patient pathological phenotypes, such as the elderly, those with CKD, and non-obese patients, are often excluded from RCTs [11]. The objective of this study was to compare the real-world effectiveness of long-acting once-weekly dulaglutide 1.5 mg injections with daily insulin injections, in patients with T2DM, by comparing change in HbA1c, weight and incidence of hypoglycaemia over 6 months.
Methods
Study Design and Setting
This was a retrospective, non-randomised, observational cohort study using data from a chart review of patients with T2DM who initiated either dulaglutide or insulin at a single private endocrinology clinic in Sydney, Australia. Data were collected from electronic medical records of patients with T2DM who were initiated on insulin between October 2010 and May 2017, and patients who were initiated on dulaglutide between May 2018 and October 2019. Ethics approval was obtained through the University of Notre Dame Australia Human Research Ethics Committee (HREC) in accordance with the National Statement on Ethical Conduct in Human Research (2007, updated 2018) in September 2019.
Participants
A chart review was performed and patients with suboptimal glycaemic control (HbA1c > 7%), who needed the greater glucose-lowering effect of an injectable AHG drug, were included in the analyses. All patients were referred to the clinic, a secondary healthcare facility, by their primary healthcare provider for expert management of their T2DM. All patients met criteria for reimbursement of their AHG injectable therapies (dulaglutide, basal or premix insulin) under the Australian national Medicare Pharmaceutical Benefits Scheme (PBS). Patients were selected consecutively from the clinic database until 75 patients were reached in each treatment cohort. The data from patients were included in the analyses if they were naïve to routine injectable therapy. However, patients were excluded if they had temporarily been prescribed insulin therapy to treat reactive hyperglycaemia in hospital within 3 months prior to commencing regular outpatient insulin therapy. All patients were receiving at least one oral AHG therapy (see Table 1).
Patients self-administered their prescribed injectable therapy as part of their normal medication routine, subsequently reporting adherence at follow-up visits. Insulin was either basal (U100 glargine) or premix (biphasic insulin aspart). Insulin was prescribed according to the patients’ clinical needs and titrated to target on the basis of the progress of self-monitored blood glucose. Patients were comanaged with a credentialed diabetes educator (CDE) to aid insulin titration (see Table 2 for titration regime).
Dulaglutide was prescribed as a 1.5 mg weekly subcutaneous injection. In Australia, dulaglutide was first available for treatment of T2DM in May 2018 (via an early access program a month before its listing on the PBS) and only available at the 1.5 mg dose so there was no dose titration involved. Measurements had been recorded in each patient’s chart at baseline (visit 1, initiation of the injectable) and at two follow-up visits (visit 2 and visit 3). The first follow-up was at approximately 3 months (± 1 month) after initiation and the second follow-up was at approximately 6 months (± 1 month) after initiation.
Outcome Measures
Patient demographic and clinical characteristics that had been collected in the patient chart at baseline (visit 1) included ethnicity, sex, age, weight, blood pressure, duration of diabetes, HbA1c (%), serum creatinine, estimated glomerular filtration rate, serum lipid profile and urine albumin/creatinine ratio. All concurrent oral AHG therapies, including changes to dosage across visits for metformin, sulfonylureas, dipeptidyl peptidase 4 (DPP4) inhibitors and SGLT2 inhibitors, were also recorded. The presence of previous and current diabetic complications was recorded at each visit, including macrovascular disease (ischaemic heart disease (IHD), cerebrovascular accidents, peripheral vascular disease) and microvascular disease (diabetic neuropathies, nephropathy and retinopathy).
Measures of glycaemic control included change in HbA1c from baseline to visit 2 and visit 3 and the percentage of patients reaching a HbA1c target of ≤ 7% at visit 2 and visit 3. Changes in weight were assessed from baseline to visit 2 and visit 3. The incidences of hypoglycaemia were reported to the clinician by the patients at each visit. A blood glucose level ≤ 3.9 mmol/L recorded on the patient’s blood glucose monitor or documented in a diary would satisfy criteria for a hypoglycaemic event.
Data Sources/Measurement
HbA1c was measured using a National Glycohemoglobin Standardization Program (NGSP) certified method. HbA1c values reported in NGSP units (%) were converted to International Federation of Clinical Chemistry (IFCC) units (mmol/mol) using the following equation: 10.929 × [HbA1c (%) – 2.15] [12]. A hypoglycaemic event was defined as a blood sugar level of less than or equal to 3.9 mmol/L as measured with a blood glucose meter by the patient in between visits with or without the patient reporting a clear hypoglycaemic symptomatology (presyncope, shakiness, diaphoresis, visual disturbance, nausea, confusion) [13]. The weight of the patient was measured at each visit on the same set of scales at the clinic, with patients fully-clothed but without wearing shoes.
Sample Size
The study was designed with 80% power to show a difference in the proportion of patients achieving HbA1c < 7% at 12 months of dulaglutide 1.5 mg versus insulin and a one-sided α of 0.05, assuming at least 20% difference between treatments. This corresponded to approximately 75 patients per cohort. Assumptions for the HbA1c were based on the AWARD-2 trial [14].
Statistical Methods
All variables were summarised using descriptive statistics. Mean, standard deviation (SD), median, minimum, and maximum were reported for continuous variables and counts and percentages for categorical variables. Differences in the baseline characteristics among the dulaglutide and insulin cohorts were assessed using the Fisher's exact test, Welch two-sample t test or the Wilcoxon rank sum test. As a result of the observational nature of the study and the lack of randomisation of patients into the two study cohorts, augmented inverse probability weights (AIPW) were used to perform doubly robust estimations of the average treatment effect (ATE); 1000 bootstrap samples were used to calculate standard errors. Variables used in the propensity score, fitted using logistic regression, included age, weight, creatinine, past/present IHD, metformin use and HbA1c. To assess whether balance was achieved by the weights, standardised difference (acceptable ranges are < 0.25 or < 0.1) and variance ratio (acceptable ranges 0.5–2.0) statistics were calculated. For sensitivity analysis, the greedy 1:1 matching algorithm, calliper = 0.2 × SD[logit(propensity score)] was investigated; however, the effective sample size reduced by approximately 30 so it was not considered as part of the final analysis. No imputation of missing data was conducted. All analyses were conducted using SAS 9.4 implemented using PROC PS MATCH and PROC CAUSALTRT (SAS Institute, Cary, NC, USA).
Results
Baseline Characteristics
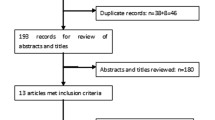
A total of 150 patients (75 initiating dulaglutide and 75 initiating insulin) were identified from the chart review that met the inclusion/exclusion criteria.
Table 1 shows the baseline characteristics of the patients in the study. Most patients were male (66%) and of Arab origin (80%). Patients on average (SD) were 62.3 (10.4) years of age, weighed 91.6 (18.7) kg, with a diabetes duration of 14.0 (6.7) years. The mean (SD) HbA1c was lower (8.5 (1.30)%, 69 (9) mmol/mol) in the dulaglutide group compared to the insulin group (9.6 (1.50)%, 81 (7) mmol/mol). There was also a notable difference in the use of SGLT2 inhibitors (77.3% vs 9.3%) and in the proportion of patients with IHD (5.3% vs 42.7%) at baseline between the two cohorts. There were also differences between the two cohorts in weight, metformin and DPP4 inhibitor use at baseline. Only the above variables that were considered clinically relevant and/or significant were adjusted for in the propensity score model. Table 3 shows an example of the covariate differences for the propensity score model for HbA1c ≤ 7% between visits 2 and 3.
We did not adjust for the difference in SGLT2 inhibitor use between the two cohorts. This was because SGLT2 inhibitors were not available in Australia until 2015 and so most patients that commenced dulaglutide in 2018 were already taking an SGLT2 inhibitor for glucose lowering and/or indicated need for cardiovascular or renal protection. The insulin-treated patients in this analysis were commenced on insulin during a period when SGLT2 inhibitors were either not available or before they were recommended for concurrent use with insulin therapy. The difference in DPP4 inhibitor use was influenced by the accepted practice of patients being taken off their DPP4 inhibitor as soon as they commence a GLP-1 RA because the role of a DPP4 inhibitor becomes redundant in this setting.
HbA1c
From baseline (visit 1) to visit 3, 58.7% of patients who received dulaglutide reached a target HbA1c ≤ 7% compared to 20.0% of patients who received insulin. The AIPW adjusted average treatment effect difference in the proportion reaching HbA1c ≤ 7% at visit 3 was 21.3% (95% confidence interval (CI) 2.7–43.1%; p < 0.01) (Fig. 1). There was an adjusted mean change in HbA1c of − 1.6% for patients receiving dulaglutide compared to a mean change in HbA1c of − 0.8% for patients receiving insulin from visit 1 to visit 2 (Table 4). The AIPW adjusted average treatment effect or mean difference of HbA1c from visit 1 to visit 2 between the two cohorts was 0.8% (95% CI 0.4–1.2%, p < 0.001) (Fig. 2).
Average treatment effect (ATE) for augmented inverse probability weights (AIPW) adjusted difference in mean change from each visit for HbA1c (%) and weight (kg) (bootstrap bias corrected 95% confidence intervals). V1 visit 1 (baseline, initiation of therapy), V2 visit 2, V3 visit 3. The data for HbA1c and weight shown in the box are the adjusted mean change observed between visits
Weight
Patients in the dulaglutide group lost on average 2.4 kg from visit 1 to visit 3 compared to patients in the insulin group which gained 2.0 kg (Table 4). The AIPW adjusted average treatment effect difference from visit 1 to visit 3 between the two cohorts was 4.4 kg (95% CI 2.6–7.3 kg, p < 0.001) (Fig. 2).
Hypoglycaemia Incidence
There was a total of 12 (16.0%) hypoglycaemia events for the patients in the dulaglutide group compared to 33 (44.0%) in the insulin group from visit 1 to visit 3. The AIPW adjusted average treatment effect difference was 0.09 (95% CI − 0.01 to 0.22, p = 0.0748) from visit 1 to visit 2 and was 0.20 (95% CI 0.09–0.32, p = 0.0002) from visit 2 to visit 3.
Discussion
This real-world retrospective analysis showed that efficacy of once-weekly dulaglutide 1.5 mg was greater than daily-titrated insulin with respect to reduced HbA1c, weight loss and lower incidence of hypoglycaemic events in the dulaglutide cohort. The (1) percentage of patients achieving a HbA1c target ≤ 7.0%, (2) observed reduction in HbA1c and (3) observed weight loss for patients treated with dulaglutide and insulin were all of a similar magnitude and trend to the results observed in the 52-week AWARD-2 randomised clinical trial that investigated the efficacy and safety of once-weekly dulaglutide versus insulin in patients with T2DM [14]. The results obtained in this real-world study were also in line with results observed in other real-world studies comparing dulaglutide to insulin [15, 16]. The DISPEL real-world study was a US observational claims study that compared changes from baseline in HbA1c, for patients with T2DM newly initiating dulaglutide or insulin over a 1-year period. At 1 year, a greater reduction in HbA1c was observed for patients on dulaglutide (− 1.12%, standard error (SE) 0.05) compared to insulin (− 0.51%, SE 0.05, p < 0.01)[15]. Another study in the USA used real-world treatment results to compare changes in estimated glomerular filtration rate (eGFR) and HbA1c in patients with T2DM who initiated treatment with dulaglutide or insulin. Initiation of dulaglutide was associated with a significantly smaller decrease in eGFR and a significantly larger decrease in HbA1c (− 0.5% vs − 0.2%, p < 0.0001) [16].
Previous position statements suggested that insulin is unique among AHG therapy for its powerful AHG efficacy, particularly in patients who have very high HbA1c values [17]. Therefore, in patients who demonstrated secondary failure to oral AHG therapy, insulin was almost always recommended as the final treatment frontier. However, it has since been shown that HbA1c reduction with long-acting GLP-1 RAs are at least equivalent to that with basal insulin, irrespective of baseline HbA1c, and similarly effective in patients starting at even very high baseline HbA1c values [18].
Previous data have also indicated that patients with higher baseline HbA1c levels generally demonstrate a greater reduction in HbA1c after starting AHG therapy [19]. A meta-analysis performed in 2010 of 59 clinical trials across ten categories of glucose-lowering therapies demonstrated a positive relationship between baseline HbA1c and the magnitude of HbA1c change: the higher the HbA1c at baseline, the greater the magnitude of HbA1c reduction [20]. However, in this study, the dulaglutide group demonstrated a greater magnitude of reduction in HbA1c than the insulin group, despite the fact the patients in the dulaglutide arm had a lower HbA1c at baseline compared with the patients in the insulin arm. This is very encouraging from a clinical practice perspective, especially given the equally long-standing duration of diabetes in both cohorts (14 years (SD 6.7)). Moreover, the initial HbA1c reduction achieved was maintained with dulaglutide therapy out to the end of the study (approximately 6 months). A possible explanation for this is the contribution of dysregulated glucagon secretion to hyperglycaemia in some patients with T2DM. Apart from their glucose-dependent insulinotropic action and reduction in rate of gastric emptying, the other mechanism by which GLP-1 RAs reduce plasma glucose levels in patients with T2DM (and as a point of difference to insulin therapy) is via suppression of glucagon hypersecretion. GLP-1 is known to reduce glucagon secretion in the presence of hyperglycaemia, reducing both fasting and post-prandial hyperglycaemia [21, 22]. This effect of GLP-1 RAs controlling hyperglycaemia by suppressing hyperglucagonaemia may be a point of difference for some patients regardless of baseline HbA1c and duration of T2DM, as insulin therapy only addresses beta cell but not alpha cell dysfunction. Exogenous insulin fails to completely suppress glucagon secretion in patients with T2DM [23].
Furthermore, the reduction in hypoglycaemic incidence in the dulaglutide arm can be explained two ways: firstly, insulin is a known powerful AHG agent which commonly causes hypoglycaemia; secondly, recent studies in mouse models have shown that GLP-1 can be glucagonotropic when glucagon secretion is needed during hypoglycaemia. Therefore, GLP-1 not only suppresses glucagon secretion during hyperglycaemia but stimulates glucagon secretion when it is needed at very low glucose levels which can explain the clinical observation that patients treated with GLP-1 RAs have reduced hypoglycaemic events [24].
The patients in this real-world study of dulaglutide were predominantly a Middle Eastern population. This is the first time to our knowledge that dulaglutide has been evaluated in this patient demographic.
The study had some limitations. The non-randomised design of the study meant that limited confounders were collected which may have impacted treatment choice. There is an uncertainty in unmeasured confounding factors on the average treatment effect. There is a lack of generalisability in the results because of the single-site nature of the study and its small sample size. Weight loss analysis needed to be done in kilograms instead of body mass index (BMI), as height data was not available for all of the patient population. Hypoglycaemia and therapy adherence data were dependent on patient self-reporting, which may have been unreliable at times. Dulaglutide is available to patients as an easy-to-use autopen with a pre-attached hidden needle and a simple injection technique that does not require resuspension or dose titration. Injection-naïve patients were trained on how to administer their dulaglutide injection by the treating physician during their consultation. This likely improved not just patient acceptance of injectable therapy requirement but also led to better adherence and compliance compared to daily insulin injections. Insulin therapy, on the other hand, required more intensive education by a CDE followed by up-titration of the starting dose on the basis of reported self-monitored blood glucose data which guided insulin adjustments to help reach blood glucose targets. The success of ongoing insulin titration depends upon either patient adherence to a self-titration algorithm (this was utilised in some of the patients) or patients adhering to advice from a CDE for follow up insulin titration and stabilisation. Most of the patients had a CDE incorporated in their management plan as well as the treating endocrinologist. For some patients there was difficulty implementing the ongoing communication that was required after insulin initiation in order to provide appropriate titration of insulin to reach blood glucose targets.
We compared the utility of dulaglutide versus insulin in patients needing the greater glucose-lowering effect of an injectable in whom oral AHG was no longer adequately controlling hyperglycaemia. Since carrying out our study, guidelines have been altered to reflect evolving clinical trial data. In particular, GLP-1 RAs have since been upgraded from being a less commonly used second-line approach to now being included in the usual therapeutic second-line treatment strategy, as reflected in the 2020 update of the Australian Diabetes Society (ADS) algorithm for the management of T2DM [25]. These and other guidelines now recommend that GLP-1 RAs be considered as a second-line option in patients who particularly require weight loss/BMI stabilisation, or in patients who are either at risk of, or have established, cardiovascular disease (independent of HbA1c) [26]. This is because GLP1-RAs have been shown to be associated with improved cardiovascular outcomes [8–10, 27].
Conclusions
Our results support previous findings that GLP-1 RA therapy is a powerful AHG agent which lowered HbA1c at a greater magnitude than insulin. This was despite our dulaglutide cohort having a lower baseline HbA1c, while still demonstrating positive weight loss and a reduction of hypoglycaemia incidence. The beneficial effects and therapeutic benefits of dulaglutide therapy previously observed in RCT cohorts can be translated into the real-world setting for patients requiring the greater glucose-lowering effect of an injectable. The data from this retrospective analysis support current international and Australian consensus guideline recommendations with respect to a GLP-1 RA being the preferred first injectable [3].
References
McGill JB. The SGLT2 inhibitor empagliflozin for the treatment of type 2 diabetes mellitus: a bench to bedside review. Diabetes Ther. 2014;5(1):43–63.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–24.
Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab. 2017;19(2):216–27.
Thomas M. GLP-1 receptor agonists—conceptualising a new approach to diabetes management. Australian Diabetes Educator. 2015;18(3):40–3.
Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–17.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30.
Morieri ML, Frison V, Rigato M, et al. Effectiveness of dulaglutide in the real world and in special populations of type 2 diabetic patients. J Clin Endocrinol Metab. 2020;105(7):e2617–25.
EBMCalc An Educational Medical Reference. Glycemic assessment: A1C to average glucose conversions. https://ebmcalc.com/GlycemicAssessment.htm. Accessed 11 Aug 2021.
Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–95.
Giorgino F, Benroubi M, Sun J-H, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care. 2015;38(12):2241–9.
Mody R, Huang Q, Yu M, et al. Clinical and economic outcomes among injection-naive patients with type 2 diabetes initiating dulaglutide compared with basal insulin in a US real-world setting: the DISPEL Study. BMJ Open Diabetes Res Care. 2019;7(1):e00084.
Boye KS, Mody R, Wu J, Lage MJ, Botros FT, Woodward B. Effects of dulaglutide and insulin glargine on estimated glomerular filtration rate in a real-world setting. Clin Ther. 2018;40(8):1396–407.
Gunton JE, Cheung NW, Davis TM, Zoungas S, Colagiuri S, Australian DS. A new blood glucose management algorithm for type 2 diabetes: a position statement of the Australian Diabetes Society. Med J Aust. 2014;201(11):650–3.
Buse JB, Peters A, Russell-Jones D, et al. Is insulin the most effective injectable antihyperglycaemic therapy? Diabetes Obes Metab. 2015;17(2):145–51.
Jones AG, Lonergan M, Henley WE, Pearson ER, Hattersley AT, Shields BM. Should studies of diabetes treatment stratification correct for baseline HbA1c? PLoS ONE. 2016;11(4):e0152428.
DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trials. Diabet Med. 2010;27(3):309–17.
Holst JJ, Christensen M, Lund A, et al. Regulation of glucagon secretion by incretins. Diabetes Obes Metab. 2011;13(Suppl 1):89–94.
Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev. 2005;21(2):91–117.
Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4–12.
Zhang Y, Parajuli KR, Fava GE, et al. GLP-1 Receptor in pancreatic alpha-cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes. 2019;68(1):34–44.
The Royal Australian College of General Practitioners. Management of type 2 diabetes: a handbook for general practice. East Melbourne: RACGP; 2020.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221–8.
Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29.
Acknowledgements
Funding
This was an investigator initiated trial which has received support from Eli Lilly by way of statistical support. The journal’s Rapid Service Fee was also supported by Eli Lilly.
Medical Writing Assistance
The authors thank Theresa Wade, PhD of WriteSource Medical Pty Ltd, Sydney, Australia, for providing medical writing services funded by Eli Lilly Australia in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
JH contributed to conceiving the study, collecting the data, and contributed to data analysis. AB analysed the data. MO conceived the study and provided access to the data. All authors contributed to drafting of the manuscript.
Prior Presentation
A poster of the results of this research was presented at the Australasian Diabetes Congress in November 2020.
Disclosures
Jared Houssarini has no competing interests to declare. Alan Brnabic is an employee and shareholder of Eli Lilly Australia Pty Ltd. Marwan Obaid has received honoraria for speaking engagements with Eli Lilly, Boehringer Ingelheim, AstraZeneca, Novo Nordisk and Sanofi and has participated in advisory board meetings with Eli Lilly, AstraZeneca, Novo Nordisk and Sanofi.
Compliance with Ethics Guidelines
Ethics approval was obtained through the University of Notre Dame Australia Human Research Ethics Committee (HREC) in accordance with the National Statement on Ethical Conduct in Human Research (2007, updated 2018) in September 2019. This study was deemed low risk owing to the anonymised and retrospective nature of the data, and hence the requirement to obtain consent was waived.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Houssarini, J.A., Brnabic, A.J.M. & Obaid, M. Comparing Real-World Effectiveness of Dulaglutide and Insulin as the First Injectable for Patients with Type 2 Diabetes: An Australian Single-Site Retrospective Chart Review. Diabetes Ther 13, 131–144 (2022). https://doi.org/10.1007/s13300-021-01184-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01184-x