Abstract
Introduction
The latest Position Statement of the American Diabetes Association/European Association for the Study of Diabetes proposes the use of a fixed-ratio combination (FRC) of a long-acting basal insulin and a glucagon-like peptide-1 receptor agonist as part of treatment intensification. This study aimed to assess the effectiveness of the insulin glargine + lixisenatide (iGlarLixi) FRC on glycaemic control and hypoglycaemia in real-life settings.
Methods
This non-interventional, 26-week study included participants aged 18–80 years with suboptimally controlled type 2 diabetes (T2D) using oral antidiabetics (OADs) ± basal insulin therapy. The primary efficacy endpoint was the proportion of participants who achieved at least a 1% decrease in glycated haemoblobin (HbA1c) level from baseline to week 26.
Results
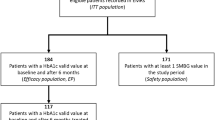
Of the 441 participants eligible for entry into the study, 353 were included in the efficacy analyses. These individuals were switched from OADs without (282 [79.9%]) or with (71 [20.1%]) insulin-based treatment. A reduction in HbA1c of at least 1.0% (primary endpoint) was achieved by 215 subjects (60.9%). All glycaemic variables (mean ± standard deviation) improved significantly during follow-up (HbA1c, from 8.9 ± 1.31 to 7.4 ± 0.97%; fasting blood glucose, from 9.0 ± 2.18 to 6.9 ± 1.23 mmol/L; postprandial blood glucose, from 11.3 ± 2.33 to 8.5 ± 1.46 mmol/L; p < 0.001 for all). Body weight also decreased during follow-up, from 90.5 ± 18.03 to 88.2 ± 17.75 kg (p < 0.001). Overall, 41 participants (9.3% of the safety population) self-reported 101 non-severe hypoglycaemic episodes (incidence rate 0.498 events/person-year). There were no severe hypoglycaemic episodes reported. Gastrointestinal adverse events were reported by five participants (1.1% of the safety population). The vast majority (96.6%) of the study population continued iGlarLixi treatment after the final visit.
Conclusion
The results of this non-interventional study confirmed the efficacy results of the randomized controlled trial programme of the iGlarLixi FRC in a real-life setting. iGlarLixi significantly improved glycaemic control in association with a low frequency of hypoglycaemia and gastrointestinal adverse events in a heterogeneous population of participants with T2D suboptimally controlled with OADs ± basal insulin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Fixed-ratio combinations (FRC) of a long-acting basal insulin and a GLP-1RA (including the FRC of insulin glargine + lixisenatide [iGalrLixi]) are a newly proposed treatment modality in patients with diabetes who require treatment intensification. |
The aim of this observational study was to assess the efficacy and safety of the iGlarLixi FRC in real-world settings. |
What was learned from the study? |
All glycaemic variables and body weight significantly improved and no serious adverse events were detected. |
The efficacy outcomes confirmed the initial hypothesis that the iGlarLixi FRC could significantly improve glycaemic control overall and in subgroups of the patients. |
This is the first real-life study of iGlarLixi FRC in Hungary, with data supporting the results of the LixiLan randomized controlled trials. |
Introduction
The Position Statement in the latest Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes in 2018 (ADA-EASD) [1] recommends changing the treatment of patients with diabetes when dual/triple combinations prove inefficient, shifting focus from intensified insulin therapy to a therapeutic regimen consisting of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in combination with metformin. If the treatment needs to be intensified still further, and insulin has to be added to the regimen, the Position Statement proposes that physicians should consider a fixed-ratio combination (FRC) of a long-acting basal insulin and a GLP-1RA [1].
The superiority of the insulin glargine + oral antidiabetics drugs (OADs) combination over the neutral protamine Hagedorn (NPH) insulin + OAD combination or premixed insulin preparations has been demonstrated [2]. GLP-1RAs belong to a relatively new class of antidiabetic drugs and have in recent years become an integral part of the treatment of type 2 diabetes (T2D). The Position Statement [1] highlighted the beneficial effects of specific GLP-1RAs on cardiovascular outcomes as well as in secondary prevention for patients with established cardiovascular disease.
GLP-1RAs and basal insulin may well complement each other due to their different mechanisms of action. The effects of short-acting GLP-1RAs predominantly affect the postprandial glucose (PPG) peak [1, 3]. Combinations of GLP-1RAs + basal insulin target seven of the eight metabolic abnormalities that characterize T2D [4]. In addition, GLP-1RAs can reduce food intake and consequently counteract the effects of insulin on body weight gain. Administering insulin and GLP-1RAs as titratable fixed-ratio co-formulations provides additional benefits, such as less complex administration, more convenient and flexible dosing schedule and fewer injections in a day. In one study, the administration of basal insulin + GLP-1RAs as FRCs achieved the same glycaemic control as when each of the components was administered alone—but at lower doses [4].
Lixisenatide was investigated in the extensive ‘GetGoal’ development programme [3] and was demonstrated to improve glycaemic control compared to placebo. The once-daily titratable basal insulin glargine 100 units/mL + GLP-1RA lixisenatide (iGlarLixi) FRC was investigated in a phase 2 study [5] and in the LixiLan drug development programme (that consisted of three phase 3 clinical trials: LixiLan-O [6], LixiLan-L [7] and LixiLan-G [8]). The LixiLan-O [6] study included people suboptimally controlled with OADs. The results demonstrated that iGlarLixi significantly improved glycated haemoglobin (HbA1c) levels between baseline and week 30, compared with participants receiving the components separately, with those receiving the FRC achieving a significantly greater decrease in HbA1c levels. iGlarLixi was also superior in terms decreasing body weight compared to iGlar. The LixiLan-L study [7] included patients inadequately controlled with basal insulin ± OADs. The results of this trial demonstrated that iGlarLixi was superior in decreasing HbA1c, fasting plasma glucose (FPG) and PPG levels as well as residual hyperglycaemia compared to iGlar alone [9]; these beneficial effects were independent of T2D duration [10]. iGlarLixi treatment in the LixiLan-L study was associated with better fasting lipid profiles compared to iGlar treatment alone [11]. When participants in the LixiLan-L study [7] randomized to iGlarLixi were compared with participants randomized to basal-bolus regimen in the GetGoal Duo-2 study [12] using propensity score matching analysis, the former treatment achieved a significantly greater improvement in HbA1c levels, as well as a lower incidence rate of hypoglycaemia and lower weight loss [13]. In both the LixiLan-O and LixiLan-L studies, iGlarLixi was shown to be superior in improving glycaemic variability compared to the study-specific comparator treatments (iGlar and Lixi in the former study; iGlar in the latter study) [14]. In another study, iGlarLixi achieved greater glycaemic control than iGlar with a similar rate of hypoglycaemic events and was associated with less gastrointestinal adverse events than lixisenatide alone [15]. iGlarLixi has also been shown to perform well in patients aged > 65 years [16]: in addition to achieving greater improvement in glycaemic status than comparators, iGlarLixi reduced the weight gain effect of insulin and gastrointestinal events associated with lixisenatide in this population. In the extension of the LixiLan-G trial [17], patients randomized to iGlarLixi maintained the glycaemic control that was achieved at 26 weeks and had a similar safety profile at weeks 26 and 52. Furthermore, a meta-analysis based on the iGlarLixi and iDegLira drug development studies demonstrated that FRCs had more favourable effect on body weight than basal insulin alone in patients switching from basal insulin to a FRC [18].
As several routine practical questions have been raised since the introduction of FRCs that could not be addressed based merely on the ADA-EASD Position Statement and the Summary of Product Characteristics (SmPC), Central and Eastern European experts published consensus opinions related to the daily use of iGlarLixi [19]. These covered questions such as sequential versus simultaneous initiation of insulin glargine and lixisenatide, de-intensification from the basal-bolus insulin regimen to iGlarLixi, discontinuation of OADs when initiating the iGlarLixi FRC and the advantages of iGlarLixi injection pens. Skolnik et al. [20] have demonstrated the applicability of iGlarLixi in real-world cases.
Despite the thorough randomized controlled trial (RCT) programme as well as the expert opinion consensus, there is current no real-world evidence on the effectiveness and safety of the iGlarLixi FRC. In Hungary, the iGlarLixi FRC was marketed in 2017 as Suliqua® (the European brand name for iGlarLixi; Sanofi-Aventis, Paris, France) and in two different ratio combinations: 100 U/mL insulin glargine + 50 U/mL lixisenatide (Suliqua® SoloStar pen 10–40 U; hereafter referred to as ‘Suliqua 100/50’) and 100 U/mL insulin glargine + 33 U/mL lixisenatide (Suliqua® SoloStar pen 30–60 U; hereafter referred to as ‘Suliqua 100/33’) [21]). The primary aim of our single-arm, non-interventional study was to assess the effectiveness of the iGlarLixi FRC on glycaemic control in people with T2D who switched from an OAD or basal insulin + OAD treatment regimen.
Methods
This retrospective/prospective non-interventional study included people with T2D who were 18–80 years old;treated with one or more OADs with or without basal insulin for at least 3 months immediately prior to enrolment; had uncontrolled glycaemic status despite current treatment regimen (defined as HbA1c > 7.5%); and had signed the patient information leaflet and the statement of consent. People were excluded if they used a GLP-1RA ≤ 3 months prior to enrolment; had used any prandial or premix insulin within 6 months prior to enrolment; were known to be allergic to any components of Suliqua®; and met any the contraindications in the Suliqua® SmPC. Most of the participants were included in a prospective manner at the initiation of iGlarLixi; however, based on an approved protocol amendment, some participants were included shortly after the initiation of iGlarLixi, in a retrospective manner.
In accordance with the study design, iGlarLixi was prescribed based on the SmPC, local and international recommendations and the national reimbursement rules. The decision to start iGlarLixi treatment and the titration of iGlarLixi were the responsibility of the investigator and was independent of the patient’s participation in this study. The participants were selected from among those whom the investigator had prescribed iGlarLixi.
In order to determine the sample size, we hypothesized that mean reduction in HbA1c would be 1.0% (based on the results of LixiLan-O study [6]), and 50% of patients achieved this reduction. To be able to detect this ratio with an accuracy of ± 5% with a 95% confidence interval (CI), 385 patients needed to be analysed, while the detection of lower or higher ratios required fewer patients.
The primary efficacy endpoint was the proportion of participants whose HbA1c level decreased by at least 1.0% from baseline to the end of the study. The secondary efficacy endpoints were related to changes in glycaemic variables, body weight and doses of iGlarLixi during the study period. The safety endpoints included the incidence of hypoglycaemic and other adverse events. Study-related data were collected at baseline (at participant inclusion, visit 1), at 13 ± 4 weeks (visit 2) and at 26 ± 6 weeks (visit 3), according to standard patient care.
The safety population included all participants who had minimum of one data item that was recorded in addition to the data at the baseline visit. The efficacy population included participants who had HbA1c endpoint data at baseline and at week 26 ± 6 (visit 3).
Data are presented as the mean ± standard deviation (SD) or 95% CI for continuous variables and as the number and proportion for categorical variables. Normal distribution of continuous variables was tested using a quantile–quantile plot. The paired t test was used for testing changes in time, the chi-square test for comparing frequencies and analysis of variance for comparing continuous variables in more than two groups. Statistical significance was defined at a level of p < 0.05. All analyses were run in IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA).
The study conformed to the Helsinki Declaration of 1964, as revised in 2013, and was approved by the Hungarian National Institute of Pharmacy and Nutrition (registration code: OGYÉI/21925-1/2018). All participants provided written informed consent before collection of any data. The first version of the protocol was modified on 29 September 2019 to enable additional retrospective data to be collected.
Results
Study Population and Baseline Characteristics
Of the 441 participants included in the study, all met the criteria of ‘safety population’ and 353 (79.8%) met the criteria of ‘efficacy population’. All 25 participants who were enrolled in a retrospective manner (according to the study protocol amendment) were included in the efficacy population as they met its criteria.
The demographic and baseline characteristics of the efficacy population are summarized in Table 1. The mean (± SD) age of the participants was 51.3 ± 9.73 years at the time of T2D diagnosis and 61.4 ± 10.25 years at baseline; the mean duration of T2D was 10.1 ± 6.7 years at enrolment. In total, 46.7% of the participants were male. Baseline glycaemic values (HbA1c 8.9 ± 1.31%, FPG 9.0 ± 2.18 mmol/L, PPG 11.3 ± 2.33 mmol/L) indicated suboptimal control of T2D.
Most participants (N = 282 [79.9%]) were switched from OAD-only regimens; the remaining participants (N = 71 [20.1%]) were switched from an insulin-based treatment, namely human basal insulin (17 [4.8%] and basal insulin analogues (54 [15.3%]) (Table 1). Baseline HbA1c levels in participants switched from OAD-based regimens were significantly higher than those in participants switched from insulin-based regimens (9.1 ± 1.36 vs. 8.2 ± 0.77%; p < 0.001).
The investigators specified the titration range of FPG for 328 participants (lower and upper limits: 5.5 ± 0.70 and 6.8 ± 0.77 mmol/L, respectively) and the target HbA1c value for 326 participants (mean ± SD target: 7.0 ± 0.39%; target of ≤ 7.0% for 79.7% of participants) (Table 1).
Primary Efficacy Endpoint
In the efficacy population, 215 subjects (60.9%; 95% CI 55.6%, 66.0%) attained ≥ 1% point decrease in HbA1 level between baseline and week 26.
Secondary Efficacy Endpoints
Changes in Glycaemic Variables
All three main glycaemic variables (Table 2) decreased significantly from baseline to week 13 ± 4 and 26 ± 6 (mean [95% CI]: HbA1c 1.3 [− 1.42, − 1.13] and − 1.6 [− 1.70, − 1.41], respectively; FPG: − 1.8 [− 2.03, − 1.56] and − 2.1 [− 2.32, − 1.85], respectively; PPG: − 2.5 [− 2.74, − 2.23] and − 2.8 [− 3.07, − 2.56], respectively; p < 0.001 for all changes; see Fig. 1a). By week 13 ± 4 and week 26 ± 6, 21.0% (N = 73) and 34.8% (N = 123) of participants, respectively, had achieved the target range of HbA1c < 7% (Table 2).
a Glycaemic variables at baseline and at weeks 13 and 16 (mean with standard error). Asterisk above bar indicates a statistically significant difference (*p < 0.001) from baseline. b Change (decrease) in HbA1c (mean % with standard error in parentheses) from baseline at week 26 ± 6 (values under bars) in subgroups categorized by baseline HbA1c range (values above bars). FPG fasting plasma glucose, HbA1c glycated haemoglobin, PPG postprandial glucose
The proportions of participants who achieved HbA1c < 7% were compared according to subgroups formed on the basis of (1) experiencing any hypoglycaemic events during the study, (2) by treatment regimen before switch to iGlarLixi and (3) by participant age (see Table 2). None of the comparisons resulted in statistically significant differences in the proportions of participants achieving HbA1c < 7%. However, the rates suggested that participants with hypoglycaemic events (vs. those without hypoglycaemic events) and those switching from OAD-only regimen (vs. those switching from insulin-based regimen) achieved better glycaemic control.
The higher the baseline HbA1c levels, the greater the decrease by week 26 ± 6 (Fig. 1b): the improvements in HbA1c levels (mean % with 95% CI in parentheses) were − 0.86 (− 0.98, − 0.75), − 1.46 (− 1.64, − 1.28) and − 3.00 (− 3.33, − 2.66) in the participant subgroups with baseline HbA1c levels of > 7.5 to ≤ 8.5% (N = 150), > 8.5 to ≤ 9.5% (N = 89) and > 9.5% (N = 92), respectively (all changes between baseline and week 26 ± 6 were significant, p < 0.001). However, the changes in HbA1c levels in patients with BMI ≥ 30 and < 30 kg/m2 were not significantly different.
Changes in Body Weight and BMI
Body weight (kg, mean [95% CI]) showed a small but significant decrease from baseline to weeks 13 ± 4 and 26 ± 6 (− 1.67 [− 2.11, − 1.24] and − 2.32 [− 2.92, − 1.72], respectively; p < 0.001 for both comparisons). Sixty-five percent of the participants experienced weight loss by week 26 ± 6 (Table 2). Similarly, BMI and the proportion of patients with BMI ≥ 30 kg/m2 decreased from baseline to weeks 13 ± 4 and 26 ± 6.
iGlarLixi Treatment
The proportion of participants for whom Suliqua ‘100/33’ was prescribed increased from 4.0% at baseline to 18.6 and 22.7% at week 13 and 26, respectively.
The mean dose of insulin glargine (as a component of the iGlarLixi FRC) increased from 13.1 ± 5.47 U at baseline to 25.2 ± 10.43 U at week 13 ± 4 and 28.1 ± 11.95 U at week 26 ± 6 (doses administered at week 13 ± 4 and 26 ± 6 were significantly higher than those prescribed at baseline; see Table 1). The doses were increased in 90% of the participants, were unchanged in 8% and were decreased in 2% from baseline to week 26 ± 6. Similarly, the doses of lixisenatide were increased from 6.4 ± 2.23 μg at baseline to 11.4 ± 3.82 μg at week 13 ± 4 and to 12.4 ± 4.23 μg at week 26 ± 6 (doses at both later time points were significantly higher than the dose prescribed at baseline; see Table 1). The proportion of participants who were administered iGlarLixi in the morning or at lunch or dinner did not change from baseline to week 26 ± 6 (from 42.2 to 44.5%, from 16.4 to 17.3%, and from 41.4 to 38.0%, respectively). The investigators found that iGlarLixi treatment was continued after the end of the study in majority (96.6%) of the participants.
Secondary Safety Endpoints
Hypoglycaemic Events
Between the baseline and week 26 ± 6 visits, 101 non-severe hypoglycaemic events were reported in 41 participants (9.3% of the safety population), which corresponded to an incidence rate of 0.498 events/person-year. When the two study periods (i.e. from baseline to week 13 ± 4 and from week 13 ± 4 to week 26 ± 6) were compared, a statistically significant difference was noted between the incidence rates of non-severe hypoglycaemic events (0.667 and 0.404 events/person-year, respectively; p = 0.0107) (period 1: 69 events in 29 participants [6.6%]; period 2: 40 events in 23 participants [5.2%]). No differences in incidence rates were detected between participants achieving versus those not achieving the primary study endpoint, i.e. a minimum decrease of HbA1c of at least 1% (0.512 vs. 0.377 events/person-year; p = 0.1778).
Seven nighttime (from bedtime to waking according to normal daily routine) non-severe hypoglycaemic events were reported by two participants (0.5% of the participants, 0.035 events/person-year).
No severe hypoglycaemic event was reported during the study.
Other Adverse Events
During the entire study period, 13 adverse events were reported among 11 participants (2.5%). Of these, five events (in 5 participants) were classified as serious; three of these participants died (due to accident, pneumonia and heart attack, respectively), but the deaths were not considered to have a causal association with the use of iGlarLixi or the study procedures. Five of the 13 adverse events were gastrointestinal (in 5 participants), with two resulting in permanent treatment or study discontinuation; none were classified as serious.
Discussion
One of the most important recent changes in the treatment paradigm of T2D is the shift from intensified insulin therapy to GLP-1RAs in combinations with metformin when dual or triple combinations prove inefficient or even after a single OAD when cardiovascular prevention is the primary aim. If the treatment needs to be intensified further by adding insulin to the therapeutic regimen, then physicians should consider an FRC of a long-acting basal insulin and a GLP-1RA [1].
Current treatment practices utilize the complementary effects of insulin and GLP-1RAs at several points of metabolism, which include the inhibition of glucose production in the liver, stimulation of glucose-dependent insulin secretion and delaying action on gastric emptying [4], with the aim of achieving a higher treatment efficacy for glycaemic control. Using these two medications in co-formulations results in a more convenient dosing schedule and fewer injections per day. The iGlarLixi and lixisenatide FRC has beens demonstrated to be effective in participants who had previously received various treatment regimens and in comparison to different treatments [5,6,7,8, 18].
The objective of the present non-interventional study was to collect real-word data on the effectiveness and safety of the iGlarLixi FRC in people who were suboptimally controlled with an OAD with or without insulin. In line with the non-interventional study design, iGlarLixi was prescribed based on the decision of the participating investigators as well as according to international guidelines, the approved drug label and domestic reimbursement rules.
The participants’ baseline characteristics showed that the majority were obese. Glycaemic control before the switch to iGlarLixi was significantly better in those participants treated with regimens containing insulin than in those treated with an OAD only. The target HbA1c range was specified to be ≤ 7.0% in almost 80% of the participants for whom a target was specified (92% participants). The great majority (96%) of participants were prescribed Suliqua ‘100/50’ at the time of the switch to iGlarLixi.
More than half of the participants achieved the primary endpoint, which was ≥ 1% decrease in HbA1 levels. iGlarLixi treatment resulted in significant improvement in all glycaemic variables. The decrease in HbA1c levels was similar to that in the iGlarLixi arm of the LixiLan-O study (where the participants were previously on OADs only) [6] and greater than that in the iGlarLixi arm of the LixiLan-L study (where the participants were previously on OADs + basal insulin) [7]. On the other hand, the proportion of participants with HbA1c levels < 7% at the end of the study period was much lower in our study than in patients switching from OAD-only or insulin-based regimens in the LixiLan studies. This difference can be at least partially explained by the significantly higher baseline HbA1c levels in our study population than in the LixiLan studies in which participants received optimized treatment in a 4-week run-in period before randomization. In addition, iGlarLixi was better titrated in both the LixiLan studies, and the doses at the end of the study periods were also higher. We observed that the worse the glycaemic control at baseline, the higher the decrease in HbA1c levels (Fig. 1b), which clearly showed the efficacy of the iGlarLixi FRC under routine circumstances despite a less stringent titration than the one applied during the LixiLan studies.
The body weight of the participants in our study decreased slightly, with the reduction comparable to that detected in the LixiLan studies.
The absence of reported serious hypoglycaemic events and a significant decrease in the incidence rates of non-severe hypoglycaemic events from the first to the second study periods suggest that the investigators could safely use iGlarLixi. The considerably lower incidence rate of hypoglycaemic events in our study compared to the LixiLan studies may be explained by the real-life study setting, which consequently explains the lower level of the reporting of hypoglycaemic events, less stringent titration, lower insulin doses at the end of the study period and lower proportion of participants reaching their HbA1c target. However, the study showed that in real-life conditions, despite using a new type of antidiabetic treatment, investigators were able to obtain at least a 1% decrease in HbA1c with a low incidence of hypoglycaemic in the majority of participants.
The most important limitations of this study derive from the observational study design: lack of control group limits the evaluation of the results, and—as described above—a real-life setting is associated with less stringent titration and adverse event reporting.
Conclusions
This first European non-interventional study confirmed the efficacy results of LixiLan-O and LixiLan-L studies in a real-world setting. Switching people with suboptimally controlled T2D from OADs without or with insulin to iGlarLixi treatment resulted in a clinically significant improvement in glycaemic control. It also resulted in a low frequency of non-severe adverse events, specifically no severe hypoglycaemic events and a low incidence of gastrointestinal adverse events, in a heterogeneous population. While randomized clinical trials (RCTs) have shown the potential of iGlarLixi, the current real-life study demonstrated the efficacy of the product on a daily basis. Together, these two types of studies (RCTs and real-life studies) provide convincing evidence that treatment with iGlarLixi is simple and easy to use in a wide range of patients, as well as being effective and safe. Satisfaction with the treatment was indicated by the high percentage of participants who completed the 6-month study and by the decision of the investigators to continue iGlarLixi treatment in a great majority of participants after the end of the study period.
Abbreviations
- BMI:
-
Body mass index
- FPG:
-
Fasting plasma glucose
- FRC:
-
Fixed-ratio combination
- GLP-1RA(s):
-
Glucagon-like peptide-1 receptor agonist(s)
- iGlarLixi:
-
Insulin glargine and lixisenatide
- OAD:
-
Oral antidiabetics
- SmPC:
-
Summary of Product Characteristics
- T2D:
-
Type 2 diabetes
References
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701. https://doi.org/10.2337/dci18-0033.
Rys P, Wojciechowski P, Rogoz-Sitek A, Niesyczynski G, Lis J, Syta A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52(4):649–62. https://doi.org/10.1007/s00592-014-0698-4.
Petersen AB, Christensen M. Clinical potential of lixisenatide once daily treatment for type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2013;6:217–31. https://doi.org/10.2147/DMSO.S45379.
Blonde L, Anderson JE, Chava P, Dendy JA. Rationale for a titratable fixed-ratio co-formulation of a basal insulin analog and a glucagon-like peptide 1 receptor agonist in patients with type 2 diabetes. Curr Med Res Opin. 2019;35(5):793–804. https://doi.org/10.1080/03007995.2018.1541790.
Rosenstock J, Diamant M, Aroda VR, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof-of-concept randomized trial. Diabetes Care. 2016;39(9):1579–86. https://doi.org/10.2337/dc16-0046.
Rosenstock J, Aronson R, Grunberger G, et al. Benefits of lixilan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39(11):2026–35. https://doi.org/10.2337/dc16-0917.
Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39(11):1972–80. https://doi.org/10.2337/dc16-1495.
Blonde L, Rosenstock J, Del Prato S, et al. Switching to iGlarLixi versus continuing daily or weekly GLP-1 RA in type 2 diabetes inadequately controlled by GLP-1 RA and oral antihyperglycemic therapy: the LixiLan-G randomized clinical trial. Diabetes Care. 2019;42(11):2108–16. https://doi.org/10.2337/dc19-1357.
Morea N, Retnakaran R, Vidal J, et al. iGlarLixi effectively reduces residual hyperglycaemia in patients with type 2 diabetes on basal insulin: a post hoc analysis from the LixiLan-L study. Diabetes Obes Metab. 2020;22(9):1683–9. https://doi.org/10.1111/dom.14077.
Blonde L, Berard L, Saremi A, Huang Y, Aroda VR, Raccah D. Fixed-ratio combination of insulin and GLP-1 RA in patients with longstanding type 2 diabetes: a subanalysis of LixiLan-L. Diabetes Ther. 2020;11(4):1007–15. https://doi.org/10.1007/s13300-020-00797-y.
Giorgino F, Shaunik A, Liu M, Saremi A. Achievement of glycaemic control is associated with improvements in lipid profile with iGlarLixi versus iGlar: a post hoc analysis of the LixiLan-L trial. Diabetes Obes Metab. 2019;21(12):2712–7. https://doi.org/10.1111/dom.13857.
Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 trial. Diabetes Care. 2016;39(8):1318–28. https://doi.org/10.2337/dc16-0014.
Tabak AG, Anderson J, Aschner P, et al. Efficacy and Safety of iGlarLixi, fixed-ratio combination of insulin glargine and lixisenatide, compared with basal-bolus regimen in patients with type 2 diabetes: propensity score matched analysis. Diabetes Ther. 2020;11(1):305–18. https://doi.org/10.1007/s13300-019-00735-7.
Aronson R, Umpierrez G, Stager W, Kovatchev B. Insulin glargine/lixisenatide fixed-ratio combination improves glycaemic variability and control without increasing hypoglycaemia. Diabetes Obes Metab. 2019;21(3):726–31. https://doi.org/10.1111/dom.13580.
Frias JP, Dex T, Roberts M, Kaplan A. A review of the safety and adverse event profile of the fixed-ratio combination of insulin glargine and lixisenatide. Diabetes Ther. 2019;10(1):21–33. https://doi.org/10.1007/s13300-018-0547-5.
Handelsman Y, Chovanes C, Dex T, et al. Efficacy and safety of insulin glargine/lixisenatide (iGlarLixi) fixed-ratio combination in older adults with type 2 diabetes. J Diabetes Complic. 2019;33(3):236–42. https://doi.org/10.1016/j.jdiacomp.2018.11.009.
Blonde L, Rosenstock J, Frias J, et al. Durable effects of iGlarLixi up to 52 weeks in type 2 diabetes: the LixiLan-G Extension Study. Diabetes Care. 2021;44(3):774–80. https://doi.org/10.2337/dc20-2023.
Liakopoulou P, Liakos A, Vasilakou D, et al. Fixed ratio combinations of glucagon like peptide 1 receptor agonists with basal insulin: a systematic review and meta-analysis. Endocrine. 2017;56(3):485–94. https://doi.org/10.1007/s12020-017-1293-6.
Haluzik M, Flekac M, Lengyel C, et al. Expert opinion on the therapeutic use of the fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide: a Central/Eastern European Perspective. Diabetes Ther. 2020;11(4):1029–43. https://doi.org/10.1007/s13300-020-00777-2.
Skolnik N, Del Prato S, Blonde L, Galstyan G, Rosenstock J. Translating iGlarLixi evidence for the management of frequent clinical scenarios in type 2 diabetes. Adv Ther. 2021;38:1715–31. https://doi.org/10.1007/s12325-020-01614-5.
Suliqua Summary of Product Characteristics. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/suliqua#product-information-section. Accessed 10 May 2021.
Acknowledgements
Funding
Sanofi-Aventis Hungary provided financial support to the investigators for conducting the study and the authors for planning the study, performing the analysis and preparing the manuscript. Sanofi-Aventis Hungary funded the journal’s Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and give their approval for this version to be published.
Authorship Contributions
János Tibor Kis: Participation in planning the study, performing the analysis and preparing the manuscript. Gábor Nagy: Participation in performing the analysis and preparation of the manuscript. Gábor Kovacs: Participation in planning the study, planning the analysis and preparing the manuscript.
List of Investigators
The authors wish to thank all of the medical doctors who participated in the study: Anna Ambrusics, Marietta Baranyai, Eleonóra Beke, Ferenc Borda, Balázs Bótyik, Etelka Bujdos, Zoltán Bujtor, Gyöngyi Csécsei, István Dobó, Zsolt Domboróczki, Zsuzsanna Fehér, Mária Fehér, Csaba Hajdú, Judit Hegedüs, Zoltán Hella, Zsolt Hermányi, Gábor Holzinger, Ildikó Joó, Csilla Kádár, János Tibor Kis, Klára Kosár, Gábor Kovács, Anna Lugosi, Margit Lukács, István Móricz, Gyula Neuwirth, István Páll, Gizella Pap, Zsuzsanna Papp, Sándor Szabó, Enikő Száldobágyi, Enikő Szfárli, Ibolya Takó, Enikő Tárnok, Zoltán Taybani, Viktor Vass, Tibor Végh, Klára Vida.
Disclosures
János Tibor Kis had a contract with and received remuneration from Sanofi-Aventis Hungary for planning the study and performing the analysis. Gábor Nagy is an employee of Sanofi-Aventis Hungary and participated in performing the analysis and preparation of the manuscript. Gábor Kovacs had a contract with and received remuneration from Sanofi-Aventis Hungary for planning the study and performing the analysis.
Compliance with Ethics Guidelines
This study conformed to the Helsinki Declaration of 1964, as revised in 2013, and was approved by Hungarian National Institute of Pharmacy and Nutrition (registration code: OGYÉI/21925–1/2018). All participants provided written informed consent before collection of any data.
Data Availability
Qualified researchers may request access to patient-level data and related documents (including, e.g., the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications). Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at “https://www.clinicalstudydatarequest.com”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kis, J.T., Nagy, G. & Kovacs, G. Effectiveness of IGlarLixi, a Fixed-Ratio Combination of Insulin Glargine 100 U/mL and Lixisenatide, in People with Type 2 Diabetes. Diabetes Ther 12, 2517–2529 (2021). https://doi.org/10.1007/s13300-021-01128-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01128-5