Abstract
Introduction
The aim of this study was to clarify the efficacy and safety of metabolic surgery in Chinese patients with type 2 diabetes mellitus (T2DM) and a body mass index (BMI) of 27.5–32.5 kg/m2.
Methods
A total of 99 patients with T2DM were enrolled in this retrospective cohort study. Of these patients, 53 had a BMI of 27.5–32.5 kg/m2 and had undergone metabolic surgery (n = 21) or were on conventional antidiabetic therapy (n = 32)]; 46 had a BMI ≥ 32.5 kg/m2 and all had undergone metabolic surgery. Primary endpoints included the triple endpoint [hemoglobin A1c < 6.5%, low-density lipoprotein cholesterol (LDL-C) < 2.6 mmol/L, and systolic blood pressure (SBP) < 130 mmHg] and successful weight loss 1 year later. Remission of diabetes, glucose and lipid metabolism, medication usage, and adverse events were evaluated.
Results
Of patients with BMI 27.5–32.5 kg/m2 undergoing metabolic surgery, 33.33% achieved the composite endpoints, and 100% achieved successful weight loss. This result was similar to that in patients with BMI ≥ 32.5 and better than those with BMI 27.5–32.5 kg/m2 receiving conventional antidiabetic therapy. A significant and similar reduction in BMI, waist circumference, SBP, serum LDL-C, hemoglobin A1c, and uric acid, as well as similar frequency postoperative adverse events, were confirmed in both metabolic surgery groups. Patients with BMI 27.5–32.5 kg/m2 who had undergonemetabolic surgery showed more metabolic improvement than those only receiving medications but they experienced more adverse events.
Conclusion
A BMI cutoff of 27.5 kg/m2 for metabolic surgery may be suitable for Chinese patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Recent guidelines have begun to loosen the restrictions on body mass index (BMI) as the strongest indication for metabolic surgery, particularly in the presence of comorbidities such as type 2 diabetes mellitus (T2DM). However, evidence regarding the efficacy and safety of such surgery at lower BMIs (down to 27.5 kg/m2), especially in Asian populations, is lacking. |
The aim of this study was to compare the safety and efficacy of metabolic surgery in Chinese patients with T2DM and a BMI of 27.5–32.5 kg/m2. |
What was learned from this study? |
Most parameters—including weight loss; the triple endpoint of hemoglobin A1c < 6.5%, low-density lipoprotein cholesterol < 2.6 mmol/L, and systolic blood pressure < 130 mmHg; glycemic control; and uric acid levels—were similar among patients with T2DM who underwent metabolic surgery whether they had a BMI of 27.5–32.5 kg/m2 or a BMI of ≥ 32.5 kg/m2, but that they were greatly improved in patients with T2DM and a BMI of 27.5–32.5 kg/m2 compared with patients in the same BMI range who only received conventional antidiabetic therapy |
A BMI cutoff of 27.5 kg/m2 for metabolic surgery may be suitable for Chinese patients with T2DM |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13705762.
Introduction
Owing to the rapid increase in the number of people in the general population with obesity in the past 30 years, obesity has become a global epidemic, increasing the morbidity and mortality of metabolic syndrome, type 2 diabetes (T2DM), and cardiovascular diseases [1, 2]. A weight loss of at least 15 kg or 10% of body weight through lifestyle interventions was confirmed to be efficacious for the remission of diabetes and reduction of cardiovascular disease outcomes in the DiRECT and Look AHEAD studies [3, 4]. In the Daqing diabetes prevention study, after a 6-year lifestyle intervention that included weight loss, patients with impaired glucose tolerance had lower diabetes onset and fewer cardiovascular disease events and deaths 30 years later [5]. Antidiabetic drugs, such as glucagon-like peptide-1 receptor agonists, sodium-dependent glucose transporter-2 inhibitors, and metformin, combined with weight loss also help to prevent and mitigate T2DM and decrease cardiovascular disease events [6,7,8,9]. In short-term and long-term studies, metabolic surgery has been found to remarkably reverse diabetes in obese patients (including type I obesity), reduce the incidence of cardiovascular disease, and improve the survival of patients, with longer and effective weight loss after the operation, compared patients receiving nonsurgical treatment [10,11,12,13,14,15]. This finding means that weight control is the key point to preventing and treating T2DM and cardiovascular diseases in obesity patients. Metabolic surgery may actually be a more appropriate treatment than nonsurgical therapy when effective weight loss cannot be maintained.
In the past 30 years, the use of metabolic surgery worldwide has primarily been based on a 1991 set of recommendations from the National Institutes of Health that limit these treatments to individuals with a body mass index (BMI) of ≥ 40 kg/m2 or to those with BMI of 35–40 kg/m2 with serious obesity-related comorbidities, such as T2DM [16]. However, studies conducted subsequent to 1991 suggested that persons with a BMI of 30–35 kg/m2 were also good candidates for metabolic surgery, achieving a rapid improvement in glycemic control and cardiovascular risk factors [13, 14, 17,18,19]. All guidelines now support this lower range as well [20,21,22,23]. In 2018, the American Diabetes Association (ADA) reduced the BMI threshold by 2.5 kg/m2, resulting in a BMI cutoff range of 27.5–32.4 kg/m2 for Asian-Americans, as this population is more prone to T2DM at a lower BMI than are Western populations [23]. Because of a lack of supporting evidence concerning metabolic surgery in Chinese patients with T2DM and a BMI of 27.5–32.5 kg/m2, the Chinese Diabetes Society (CDS) stated that metabolic surgery should be conservatively recommended in this population, especially in the presence of other cardiovascular risk factors [24]. Thus, there is a need to clarify whether metabolic surgery is suitable for Chinese patients with T2DM who have a BMI of 27.5–32.5 kg/m2.
Therefore, this retrospective study sought to clarify the efficacy and safety of metabolic surgery in Chinese patients with T2DM who have a BMI of 27.5–32.5 kg/m2. To accomplish this, we compared weight loss, blood glucose control, and related cardiovascular risk factors in Chinese patients with T2DM and a BMI of 27.5–32.5 kg/m2 who had undergone metabolic surgery with both a control group that only received antidiabetic medication and a group with T2DM and a BMI of ≥ 32.5 kg/m2 that underwent metabolic surgery.
Methods
Patients
Patients aged 16–65 years with T2DM and a BMI ≥ 27.5 kg/m2 were included in the present study. Exclusion criteria were: malignant disease, serious cardiovascular disease in the previous 6 months, other contraindications for metabolic surgery, drug or alcohol abuse, uncontrolled psychiatric illness, and pregnancy or planned pregnancy.
The study received ethical approval from the Ethics Committee of the hospital and conformed to the provisions of the Declaration of Helsinki. Anonymity of all patients was preserved.
Study Design
This retrospective cohort study was conducted between April 2013 and March 2019 at the Drum Tower Hospital, affiliated with the Nanjing University Medical School, China. We collected patient data based on previous actual follow-up information. A total of 46 T2DM patients with BMI ≥ 32.5 kg/m2 and 21 patients T2DM patients with BMI 27.5–32.5 kg/m2 who received metabolic surgery in the Department of General Surgery and completed a 1-year follow-up were included in the study. Forty-six T2DM patients with BMI of 27.5–32.5 kg/m2 who only given with conventional antidiabetic therapy (medications) in the Department of Endocrinology were identified and included in the control group based on matched age, sex, and duration of diabetes.
Laparoscopic metabolic surgery, including sleeve gastrectomy and Roux-en-Y gastric bypass, was performed by a single surgeon who had > 6 years of experience performing these surgeries. Patients were followed up postoperatively at outpatient visits by a multidisciplinary team, including an experienced diabetologist, exercise physiologist, dietitian, certified diabetes nurse educator, and psychiatrist at 1, 3, 6, and 12 months after surgery based on guidelines for the surgical treatment of obesity and T2DM [25]. Patients receiving metabolic surgery were advised to take a double dose of multivitamins daily, a pill of caltrate + vitamin 600 tablet twice daily, and an alfacalcidol soft capsule 0.5 ug once daily, with the dosage to be adjusted based on the blood test results. A ferrous succinate tablet could be added based on the plasma hemoglobin and serum ferritin levels. Antidiabetic, antihypertensive, lipid-lowering drugs were adjusted based on blood glucose, blood pressure, and serum lipid levels tested at each follow-up visit [25, 26]. The diet of postoperative patients gradually transitioned from liquid to general diet [25, 26]. Exercise physiologists provided specific exercise guidance based on the physical recovery of each patient after the operation [25, 26]. In an emergency, patients could also easily contact the multidisciplinary team in real time through the WeChat group.
Patients in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group were given advice regarding antidiabetic agents and lifestyle counseling for weight loss based on guidelines for the prevention and treatment of T2DM [24]; this advice was provided by an experienced diabetologist, exercise physiologist, dietitian, and certified diabetes nurse educator in the outpatient department once every 1–3 months.
Data collected at each follow-up for all groups included weight, height, waist circumference (WC), and blood pressure measured by a certified nurse with a standard measurement method. Laboratory measurements, including lipid profile, glycated hemoglobin A1c (HbA1c), serum uric acid, and fasting blood glucose (FBG), were tested at a fasting state in the morning following a fast for 8–10 h, and medication usage was also recorded. In addition, insulin and C-peptide levels were measured at 0 and 120 min and serum glucose at 120 min after a standard meal both at baseline and 1 year later for all groups.
Study Outcomes
The primary endpoints included the triple endpoint, i.e. HbA1c < 6.5%, low-density lipoprotein cholesterol (LDL-C) < 2.6 mmol/L, systolic blood pressure (SBP) < 130 mmHg, and successful weight loss, defined as percentage excess weight loss (EWL) > 50% at the 1-year follow-up [27, 28]. Percentage EWL was calculated as [(initial weight − follow-up weight)])/ [(initial weight − ideal weight for a BMI of 25 kg/m2)] × 100%, and failure of weight loss was defined as an EWL of < 25% [27, 28]. Secondary endpoints included weight loss, glycemic and blood pressure control, insulin resistance [(homeostasis model assessment of insulin resistance HOMA-IR): (serum FBG × serum fasting insulin)/22.5], remission of T2DM with two different criteria (defined as HbA1c < 6.5% and FBG < 5.6 mmol/L, with no use of antidiabetic agents for 1 year based on the CDS guideline [25], or HbA1c < 6.5% without any antidiabetic agents for 1 year [29]), medication usage, and adverse events. Adverse events were defined as surgery-associated complications or nutrient deficiency, with surgery-associated complications including wound infection, bleeding, leak, cholelithiasis, pneumonia, dumping syndrome, and death, and nutrient deficiency including anemia, ferritin deficiency, and osteoporosis.
Statistical Analysis
SPSS version 18.0 software (IBM Corp., Armonk, NY, USA) was used by an independent statistician to analyze the data. Statistical significance was defined as P < 0.05 with two-sided tests. Normal distribution and categorical variables were described as means ± standard errors and frequencies, respectively. Primary endpoint at 1 year, remission of diabetes, and medication usage were assessed by the Chi-squared test. Differences between baseline and post-intervention within each treatment group were evaluated using the paired Student's t-test. Differences between the intervention groups after adjusting for baseline values were evaluated by analysis of covariance.
Results
In total, 21 patients (11 men) were included in the BMI 27.5–32.5 kg/m2 metabolic surgery group, 46 (16 men) in the BMI ≥ 32.5 kg/m2 metabolic surgery group, and 32 (21 men) in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group (Fig. 1). Baseline characteristics of the study patients are shown in Table 1. With the exception of a lower baseline HOMA-IR (P = 0.032) in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group, all baseline characteristics were similar between the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group and BMI 27.5–32.5 kg/m2 metabolic surgery group. Baseline clinical characteristics did not differ between the two surgery groups (Table 1).
Primary Endpoint
At 1 year, the triple endpoint was achieved by more patients in the BMI 27.5–32.5 kg/m2 metabolic surgery group than by those in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group [7 (33.33%) vs. 0 (0%); odds ratio (OR) 0.065; 95% confidence interval (CI) 0.007–0.575; P < 0.001]; the former also achieved a higher degree of EWL > 50% (21 [100%] vs. 2 [6.25%]; OR 0.063; 95% CI 0.016–0.239; P < 0.001; Table 2, Fig. 2). There was no difference in any of the components of the triple endpoint or EWL > 50% between the two surgery groups (all P > 0.05). Moreover, at 1 year, patients in the BMI 27.5–32.5 kg/m2 metabolic surgery group were more likely to achieve HbA1c < 6.5% than those in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group (80.95 vs. 12.50%; P < 0.001; Table 2).
Risk analysis of the endpoints for control of type 2 diabetes mellitus. The endpoints include the triple endpoint [hemoglobin A1c < 6.5%, low-density lipoprotein cholesterol < 2.6 mmol/L, and systolic blood pressure < 130 mmHg), excess weight loss > 50%, BMI < 24 kg/m2, diabetes remission, and use of antidiabetic medications. EWL excess weight loss
Diabetes Control and Remission of Diabetes
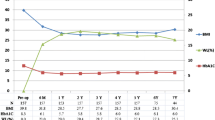
In both surgery groups, HbA1c, FBG, HOMA-IR, and serum glucose at 120 min after a standard meal significantly decreased at 1 year postsurgery (P < 0.001–0.05), but they remained high in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group (Table 1). Reductions in these values did not differ between the two surgery groups; moreover, HbA1c and FBG markedly decreased at 1 month postsurgery (P < 0.05), remaining at approximately 6% and 5–6 mmol/L, respectively, over the year of observation (Fig. 3c, d). According to the 2017 CDS guidelines [14], 33.33% of patients in the BMI 27.5–32.5 kg/m2 metabolic surgery group and 47.82% of patients in the BMI ≥ 32.5 kg/m2 metabolic surgery group achieved diabetes remission at the 1 year follow-up, with no difference in remission rates between the two surgery groups. In contrast, no patient in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group achieved this standard (Table 3). Similarly, 72.22% of patients in the BMI 27.5–32.5 kg/m2 metabolic surgery group and 69.75% in the BMI ≥ 32.5 kg/m2 metabolic surgery group attained HbA1c < 6.5% without the use of antidiabetic medications, whereas no patient in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group achieved this standard [17] (Table 3). Patients in the BMI 27.5–32.5 kg/m2 metabolic surgery group were more likely to achieve diabetes remission at 1 year than those in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group (Fig. 2).
Changes in total weight loss in the three study groups. The proportions of patients who lost at least 5, 10, or 20% of their baseline body weight in each intervention group. **P < 0.001, indicates a significant difference between the BMI 27.5–32.5 kg/m2 metabolic surgery group and the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group
Diabetes Medication
In both surgery groups, patients using antidiabetic medicines showed a similar degree of decrease 1 year later, especially in terms of insulin use (P < 0.05 for all comparisons; Table 4). Patients in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group showed an upward trend in the amount of antidiabetic medicines used (P = 0.015; Table 4). At 1 year, fewer patients in the BMI 27.5–32.5 kg/m2 metabolic surgery group than in in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group used antidiabetic drugs to control glycemia (Table 4; Fig. 2).
Weight Loss and Waist Circumference
Both surgery groups achieved significant weight loss as well as significantly decreased BMI and WC (all P < 0.001), and there were no differences between the groups. However, patients in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group did not achieve significant weight loss or a reduction in BMI or WC. Accordingly, a greater degree of weight loss and a greater decrease in BMI and WC were attained in the BMI 27.5–32.5 kg/m2 metabolic surgery group than in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group (all P < 0.001); furthermore, patients in the BMI 27.5–32.5 kg/m2 metabolic surgery group were more likely to have a BMI < 24 kg/m2 at 1 year postsurgery (Table 1; Fig. 2). The weight and BMI of the patients in the two surgery groups showed a similar downward trend at the 1 year time point (Fig. 3a, b). Rates of weight loss ≥ 10% and ≥ 20% in the BMI 27.5–32.5 kg/m2 metabolic surgery group were 100 and 81%, respectively, and in the BMI ≥ 32.5 kg/m2 metabolic surgery group, these values were 98 and 87%, respectively, at 1 year postsurgery; however, only 22% of patients in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group lost ≥ 5% of weight and no patient lost ≥ 20% (all P < 0.001; BMI 27.5–32.5 kg/m2 metabolic surgery group vs. BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group; Fig. 4).
Changes in body weight (a), BMI (b), hemoglobin A1c (HbA1c) (c), and fasting blood glucose (FBG) (d) over time in the BMI 27.5–32.5 kg/m2 metabolic surgery group and in the BMI ≥ 32.5 kg/m2 metabolic surgery group. *, **Significant difference at the indicated follow-up time-points vs. baseline at *P < 0.05 and **P < 0.001, respectively; †, ††Significant difference at the indicated follow-up time-points vs. 1 month at †P < 0.05 and ††P < 0.001, respectively; ‡, ‡‡ Signicant differences at the indicated follow-up time-points vs. 3 months at ‡P < 0.05 and ‡‡P < 0.001, respectively; §Significant difference at the indicated follow-up time-point at P < 0.05 vs. 6 months. Data are shown as the mean ± standard error of the mean
Metabolic Improvement
Systolic blood pressure, diastolic blood pressure (DBP), serum uric acid, LDL-C, and high-density lipoprotein cholesterol (HDL-C) improved markedly in the two surgery groups (P < 0.001–0.05) but did not change in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group (Table 1). Changes in SBP, DBP, serum uric acid, HDL-C, and LDL-C were not statistically significantly different between the two surgery groups. Patients in the BMI 27.5–32.5 kg/m2 metabolic surgery group achieved a higher degree of reduction in SBP, DBP, serum uric acid, HDL-C, and LDL-C than those in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group (all P < 0.05; Table 1).
Adverse Events
Significant adverse events that occurred up to 1 year after metabolic surgery or the initiation of conventional antidiabetic therapy are shown in Table 2. In the BMI 27.5–32.5 kg/m2 metabolic surgery group, patients experienced seven adverse events within 1 year, which are as follows: cholelithiasis (N = 1), anemia (N = 2), ferritin deficiency (N = 2), and osteoporosis (N = 2); this result was similar to that in the BMI ≥ 32.5 kg/m2 metabolic surgery group, but more than that in the BMI 27.5–32.5 kg/m2 conventional antidiabetic therapy group.
Discussion
Nowadays, BMI ≥ 32.5 kg/m2 serves as the threshold for recommending metabolic surgical candidacy in Chinese patients with T2DM [24, 25]. A lower BMI cutoff of 27.5 kg/m2 for Asian-Americans is recommended in Western countries [23, 30]. Although tens of millions of patients in China with T2DM have a BMI of 27.5–32.5 kg/m2, according to a guideline, metabolic surgery is a less supported option for these patients [24]. Our findings confirm that metabolic surgery resulted in better metabolic outcomes and successful weight loss at 1 year postsurgery compared to the conventional antidiabetic treatment in Chinese patients with T2DM and BMI of 27.5–32.5 kg/m2. These results are similar to those for patients with a BMI of ≥ 32.5 kg/m2 who underwent metabolic surgery.
A previous study in our hospital showed that 48% of T2DM patients with an average BMI of 33.3 kg/m2 achieved the triple endpoint, including HbA1c < 7.0%, LDL-C < 100 mg/dL, and SBP < 130 mmHg at 1 year after Roux-en-Y gastric bypass (RYGB) surgery, while only 3% of patients with an average BMI of 32.1 kg/m2 in the conventional antidiabetic therapy group achieved this goal [31]. However, this study did not clarify whether patients with lower BMI would get more benefits from metabolic surgery compared with those receiving conventional antidiabetic treatment. The Diabetes Surgery Study, a randomized clinical trial that included Asian patients, found that compared with lifestyle/intensive medical management alone, RYGB was beneficial for maintaining diabetes treatment targets, including HbA1c < 7.0%, LDL-C < 100 mg/dL, and SBP < 130 mmHg for T2DM patients with a BMI 30–35 kg/m2 at 1, 2, and 5 years postsurgery [32,33,34]. In the present study, 33.33% of patients with a BMI of 27.5–32.5 kg/m2 achieved the triple endpoints 1 year after metabolic surgery; this result was similar to that in patients with a BMI ≥ 32.5 kg/m2. In contrast, no patient with a BMI of 27.5–32.5 kg/m2 who received only conventional antidiabetic therapy achieved the triple endpoints. This strongly suggests that for T2DM patients, a BMI cutoff of 27.5 kg/m2 for metabolic surgery yields superior triple endpoint values for T2DM 1 year later, including lowering HbA1c to < 6.5%.
A number of meta-analyses that have compared metabolic surgery with medical/lifestyle treatments in T2DM patients, mostly from a Western patient population, have confirmed that metabolic surgery achieves better T2DM remission and improvement of glycemic control and HbA1c, regardless of whether the baseline BMI of a patient was below or above 35 kg/m2 [20, 29, 34, 35]. Similarly, we found that patients with a BMI of 27.5–32.5 kg/m2 who underwent metabolic surgery achieved superior glycemic control and lower HbA1c similarly to patients with a BMI of ≥ 32.5 kg/m2. The former were also more likely to achieve T2DM remission according to the strict CDS standard [25] or to relatively less strict standards [29] at 1 year after metabolic surgery than were patients with a similar BMI but who only received conventional antidiabetic therapy. Previous studies support the premise that metabolic surgery may substantially reduce the cost of antidiabetic medications and disease burden [15, 29]. In the present study, we observed that two surgery groups achieved a marked reduction in antidiabetic medications, especially insulin, while the use of antidiabetics appeared to increase in the conventional antidiabetic therapy group. Regarding patients with a BMI of 27.5–32.5 kg/m2, a higher number of those who only received conventional antidiabetic therapy used less medication to control blood glucose compared to those who underwent metabolic surgery.
Similar to prior studies [32,33,34], metabolic surgery had an advantage over antidiabetic therapy in terms of reducing body weight, BMI, and WC in T2DM patients with a BMI of 27.5–32.5 kg/m2. Weight loss and reduction of WC have been shown to improve glycemic control and metabolic disorders [34, 36]. Therefore, the greater and more sustained weight loss observed after metabolic surgery may help patients with T2DM achieve stable improvement of glycemic or lipidemic disorders or other metabolic disorders.
Consistent with other studies of outcomes following bariatric surgery for patients with T2DM with a similar or higher BMI [15, 29, 32,33,34], cardiovascular risk factors, such as high blood pressure, and serum levels of lipids and uric acid showed more favorable results in the surgical groups, including those with a BMI of 27.5–32.5 kg/m2, than in the conventional antidiabetic therapy group. Furthermore, changes in cardiovascular risk factors were comparable following metabolic surgery in patients with a BMI of 27.5–32.5 kg/m2 and those with a BMI of ≥ 32.5 kg/m2.
A systematic review and meta-analysis [29] reported that the adverse events rate of metabolic surgery for patients with a BMI 30–35 kg/m2 was 6–20% with no deaths; this value is similar to the published rates for patients with a baseline BMI ≥ 35 kg/m2 [38]. Similarly, in the present study, a similar number of surgical complications and metabolic adverse events were documented in the two metabolic surgery groups, regardless of BMI; these complications may be related to changes in anatomical structure and nutrient deficiency [39, 40]. For patients with T2DM and a BMI of 27.5–32.5 kg/m2, there were more metabolic adverse events for those who underwent surgery than for those who only received conventional antidiabetic treatment. Therefore, we believe that it is necessary to highlight the importance of postoperative follow-up and prompt use of nutritional supplements.
There are a number of limitations to our study. Firstly, it lacked an analysis of other outcomes, including diabetes complications and patient quality-of-life analyses. Second, it is a retrospective study with limited follow-up time. Finally, this study included a relatively small sample size from a single location. Trials involving larger patients sample sizes and longer duration are needed to fully evaluate the role of metabolic surgery in patients with T2DM and a BMI of 27.5–32.5 kg/m2 in the Chinese population.
Conclusions
In conclusion, the results of this study demonstrate that metabolic surgery achieves a better therapeutic triple endpoint (HbA1c < 6.5%, LDL-C < 2.6 mmol/L, and SBP < 130 mmHg) and EWL > 50% than does conventional antidiabetic treatment. They also show that metabolic surgery is associated with an improvement in metabolic disorders at 1 year postsurgery compared to the conventional antidiabetic therapy alone in Chinese patients with T2DM and a BMI of 27.5–32.5 kg/m2, and that these results were the same for patients with a BMI of > 35 kg/m2, the recognized surgical cutoff. The study provides new evidence for a BMI cutoff of 27.5 kg/m2 for metabolic surgery in Chinese patients with T2DM, thus helping extend the indication for metabolic surgery application and benefit for effective management of T2DM at a time when this disease is spreading rapidly throughout the country.
References
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396
The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27.
Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7(5):344–55.
Look AHEAD Research Group, Gregg EW, Jakicic JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016; 4(11):913–921.
Gong Q, Zhang P, Wang J, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing diabetes prevention outcome study. Lancet Diabetes Endocrinol. 2019;7(6):452–61.
Roux CWL, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–409.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications: the DPP outcomes study. Lancet Diabetes Endocrinol. 2015;3(11):866–75.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA. 2018;16;319(3):291–301.
Treatment With Long-term Medical Complications and Obesity-Related Comorbidities. JAMA 2018; 319(3):291-301
Fisher DP, Johnson E, Haneuse S, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018;320(15):1570–82.
Reis CEG, Alvarez-Leite JI, Bressan J, et al. Role of bariatric-metabolic surgery in the treatment of obese type 2 diabetes with body mass index < 35 kg/m2: a literature review. Diabetes Technol Ther. 2012;14(4):365–72.
Cummings, DE. Cohen RV. Bariatric/metabolic surgery to treat type 2 diabetes in patients with a BMI < 35 kg/m2. Diabetes care. 2016;39:924–933.
Schauer PR, Kashyap RS, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76.
[No authors listed]. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115: 956–61.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(248–256):e5.
Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–54.
Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care. 2016;39:893–901.
ASMBS Clinical Issues Committee. Bariatric surgery in class I obesity (body mass index 30–35 kg/m2). Surg Obes Relat Dis. 2013;9:e1–10.
Busetto L, Dixon J, Luca MD, et al. Bariatric surgery in class I obesity a position statement from the international federation for the surgery of obesity and metabolic disorders (IFSO). Obes Surg. 2014;24:487–519.
Aminian A, Chang J, Brethauer SA, et al. ASMBS updated position statement on bariatric surgery in class I obesity (BMI 30–35 kg/m2). Surg Obes Relat Dis. 2018;14:1071–87.
American Diabetes Association. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S81–9.
Chinese Diabetes Society. Guidelines for the prevention and treatment of type 2 diabetes in China—2017. Chin J Med Sci. 2018;38:292–344.
Liu J, Zheng C, Wang Y. Guidelines for surgical treatment of obesity and type 2 diabetes in China—2014. Chin J Pract Surg. 2014;11:1005–10.
Obesity and Diabetes Group of Chinese Diabetes Society. Chinese expert consensus on postoperative management of type 2 diabetes metabolic surgery. Chin J Diabetes Mellitus. 2018;10:161–7.
Brethauer SA, Kim J, El Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obesity Surg. 2015;25:587–606.
Suter M, Calmes JM, Paroz A, et al. A 10-year experience with laparoscopic gastric banding for morbid obesity: high long-term complication and failure rates. Obes Surg. 2006;16:829–35.
Rao WS, Xiang C, Zhang SW, et al. A Meta-analysis of short-term outcomes of patients with type 2 diabetes mellitus and BMI ≤ 35 kg/m2 undergoing roux-en-Y gastric bypass. World J Surg. 2015;39:223–30.
Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24:42–55.
Feng WH, Yin TT, Chu XH, et al. Metabolic effects and safety of Roux-en-Y gastric bypass surgery vs. conventional medication in obese Chinese patients with type 2 diabetes. Diabetes Metab Res Rev. 2019;35(5):e3138. https://doi.org/10.1002/dmrr.3138.
Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–9.
Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3:413–22.
Ikramuddin S, Korner J, Lee WJ, et al. Lifestyle intervention and medical management with vs without roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 Years in the diabetes surgery study. JAMA. 2018;319:266–78.
Douglas IJ, Bhaskaran K, Batterham RL, et al. Bariatric surgery in the United Kingdom: A cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med. 2015;12:e1001925.
Feng WH, Bi Y, Li P, et al. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: a randomized trial. J Diabetes Investig. 2019;10:399–407.
Schauer PR, Mingrone G, Ikramuddin S, et al. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care. 2016;39:902–11.
Kassir R, Debs T, Blanc P, et al. Complications of bariatric surgery: presentation and emergency management. Int J Surg. 2016;27:77–81.
Halpern B, Mancini MC. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI lower than 35 kg/m2: Why caution is still needed. Obes Rev. 2019;20:633–47.
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation of China Grant Awards (81800719, 81800752, 81970689, 81970704, and 81900787), the National Key Research and Development Program of China (2016YFC1304804 and 2017YFC1309605), the Key Research and Development Program of Jiangsu Province of China (BE2015604 and BE2016606), the Jiangsu Provincial Key Medical Discipline (ZDXKB2016012), the Natural Science Foundation of Jiangsu Province of China (BK20201115), and the Health technology development fund project of Nanjing, China (YKK18067). The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Ning-Jing Zhang, Yu-Zhe Fu, Xiao-Dong Shan, Ning Zhang, Xi-Tai Sun, Xue- Hui Chu, Yan Bi, Da-Long Zhu and Wen-Huan Feng have nothing to disclose.
Compliance with Ethics Guidelines
The study received ethical approval from the Ethics Committee of Drum Tower Hospital Affiliated to Nanjing University Medical School (approval number: 2017–030-06) and conformed to the provisions of the Declaration of Helsinki. All patient anonymity was preserved.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ning-Jing Zhang, Yu-Zhe Fu, and Xiao-Dong Shan contributed equally to this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, NJ., Fu, YZ., Shan, XD. et al. Are Chinese Patients with Type 2 Diabetes and a Body Mass Index of 27.5–32.5 kg/m2 Suitable for Metabolic Surgery? A One-Year Post-Surgery Study. Diabetes Ther 12, 1429–1444 (2021). https://doi.org/10.1007/s13300-021-01027-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01027-9