Abstract
Introduction
Type 2 diabetes represents a continuing healthcare challenge, and choosing cost-effective treatments is crucial to ensure that healthcare resources are used efficiently. The present analysis assessed the cost-effectiveness of once-weekly semaglutide 1 mg versus empagliflozin 25 mg for the treatment of patients with type 2 diabetes mellitus with inadequate glycaemic control on metformin monotherapy from a healthcare payer perspective in the UK.
Methods
Outcomes were projected over patient lifetimes using the IQVIA CORE Diabetes Model. Baseline cohort characteristics and treatment effects of initiation of once-weekly semaglutide 1 mg and empagliflozin 25 mg were based on an indirect comparison conducted using patient-level data, as there is currently no head-to-head clinical trial comparing these therapies. Modelled patients received treatments until glycated haemoglobin exceeded 7.5% (58 mmol/mol), at which point patients initiated basal insulin. The analysis captured pharmacy costs and costs of diabetes-related complications, expressed in 2019 pounds sterling (GBP). Projected outcomes were discounted at 3.5% annually. Scenario analyses were prepared to assess uncertainty around projected outcomes.
Results
Once-weekly semaglutide 1 mg was associated with increases in life expectancy and quality-adjusted life expectancy of 0.12 years and 0.23 quality-adjusted life years (QALYs), respectively, compared with empagliflozin 25 mg. Projected improvements in quality and duration of life resulted from a reduced cumulative incidence and a delayed time to onset of diabetes-related complications. Once-weekly semaglutide was associated with increased pharmacy costs, but this was partially offset by avoided costs of treating complications. Once-weekly semaglutide was associated with an increase in costs of GBP 1017 per patient, leading to an incremental cost-effectiveness ratio of GBP 4439 per QALY gained.
Conclusion
Once-weekly semaglutide 1 mg was projected to be a cost-effective treatment option from a healthcare payer perspective compared with empagliflozin 25 mg for the treatment of patients with type 2 diabetes in the UK setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Treatment guidelines released by the European Society for the Study of Diabetes (EASD) recommend the use of use of glucagon-like peptide 1 (GLP-1) receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors as second-line therapies for patients with type 2 diabetes |
There is currently no direct head-to-head trial comparing once-weekly semaglutide 1 mg and empagliflozin 25 mg, a GLP-1 receptor agonist and an SGLT2 inhibitor with evidence of a cardiovascular benefit, respectively, but a meta-analysis using individual patient data has recently been published, which has been used to inform the effectiveness parameters within this cost-effectiveness analysis |
The present analysis assessed the long-term cost-effectiveness of once-weekly semaglutide 1 mg versus empagliflozin 25 mg for the treatment of patients with type 2 diabetes mellitus with inadequate glycaemic control on metformin monotherapy from a healthcare payer perspective in the UK |
What was learned from the study? |
Once-weekly semaglutide 1 mg was associated with increases in life expectancy and quality-adjusted life expectancy of 0.12 years and 0.23 quality-adjusted life years (QALYs), respectively, compared with empagliflozin 25 mg |
Once-weekly semaglutide was associated with a mean increase in costs of GBP 1017, leading to an incremental cost-effectiveness ratio of GBP 4439 per QALY gained |
Once-weekly semaglutide 1 mg was projected to be a cost-effective treatment from a healthcare payer perspective compared with empagliflozin 25 mg for the treatment of patients with type 2 diabetes in the UK setting based on a willingness to pay threshold of GBP 20,000 per QALY gained as used by NICE |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13388420.
Introduction
It was estimated that there were 2,680,500 people living with diabetes in the UK in 2019, equating to a prevalence of 3.9%, and this is predicted to rise to 2,930,600 in 2030 (a prevalence of 4.5%) and 3,114,100 in 2045 (a prevalence of 4.8%) [1]. Estimates suggest that approximately 90% of these cases are type 2 diabetes [2]. The condition has a significant impact on mortality in the UK, with an estimated 13,951 deaths attributable to diabetes annually [1]. The disease places a large burden on healthcare payers, as it is estimated that in the UK diabetes-related healthcare expenditure was over GBP 10 billion in 2018, accounting for 10% of the entire National Health Service (NHS) budget [2]. Improving treatment for people with type 2 diabetes provides the opportunity to reduce mortality and morbidity for patients, and costs for the NHS. As the NHS budget comes under increasing pressure, it is important that therapies are both effective and cost-effective in order to deliver high-quality healthcare within constrained resources, and this is particularly important for diabetes.
Guidelines released by the European Society for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) recommend the use of glucagon-like peptide 1 (GLP-1) receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors with proven cardiovascular benefits as second-line therapies (following metformin) for patients with or at high risk of atherosclerotic cardiovascular disease, heart failure and chronic kidney disease [3, 4]. GLP-1 receptor agonists or SGLT2 inhibitors should also be considered for those with a need to minimize the risk of hypoglycaemia, and those with a need to minimize weight gain or promote weight loss, irrespective of baseline glycated haemoglobin (HbA1c) or individualized HbA1c target [3]. Once-weekly semaglutide and empagliflozin have both been shown to be associated with a cardiovascular benefit [3]. Therefore, in certain contexts clinicians may consider prescribing either once-weekly semaglutide or empagliflozin, and consequently data on the relative effectiveness and cost-effectiveness of these interventions will be important in order to make informed decisions.
To date, no head-to-head clinical trial directly comparing once-weekly semaglutide 1 mg and empagliflozin 25 mg has been conducted. Therefore a meta-analysis using individual patient data has been conducted to assess the relative efficacy of these interventions in patients with type 2 diabetes previously receiving metformin monotherapy, in line with guidance on the conduct of indirect comparisons from the National Institute for Health and Care Excellence (NICE) Decision Support Unit [5, 6]. The use of individual patient data in the meta-regression allows potential prognostic factors and effect modifiers to be adjusted at an individual patient level, potentially allowing for a better isolation of the effect of a single treatment on outcomes of interest than when aggregated (i.e. clinical trial) data are used, and this represents a key advantage of the approach used. The SUSTAIN 2 (once-weekly semaglutide versus sitagliptin), SUSTAIN 3 (once-weekly semaglutide versus once-weekly exenatide), SUSTAIN 8 (once-weekly semaglutide versus canagliflozin) and PIONEER 2 (once-daily orally administered semaglutide versus empagliflozin) clinical trials were included. The primary regression analysis included all four trials, which had durations of 52 or 56 weeks, with a complementary analysis conducted using only the 52-week trials (SUSTAIN 8 and PIONEER 2). To assess the relative efficacy of once-weekly semaglutide 1 mg and empagliflozin 25 mg, outcomes were assessed for change from baseline in HbA1c, body weight, body mass index (BMI), waist circumference, systolic and diastolic blood pressure, lipid parameters (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglycerides) and estimated glomerular filtration rate. The results were consistent in the primary and complementary analyses, indicating that the study duration did not have a significant impact on the outcomes.
The present analysis used the results from the previously published meta-analysis using patient-level data to assess the long-term cost-effectiveness of once-weekly semaglutide 1 mg versus empagliflozin 25 mg for the treatment of patients with type 2 diabetes mellitus with inadequate glycaemic control on metformin monotherapy from a healthcare payer perspective in the UK.
Methods
Modelling Approach and Overview
A cost-effectiveness analysis was performed using version 9.0 of the IQVIA CORE Diabetes Model [7]. The model is a non-product-specific diabetes policy analysis tool, capable of performing real-time simulations taking into account intensive or conventional diabetes therapy, oral hypoglycaemic medications, screening and treatment strategies for microvascular complications, treatment strategies for end-stage complications and multifactorial interventions. The model is based on a series of interdependent sub-models that simulate non-specific mortality and the complications of diabetes. Each sub-model has a semi-Markov structure and uses time, state, time-in-state and diabetes type-dependent probabilities derived from published sources. Model outputs include time to onset and cumulative incidence of complications, life expectancy, quality-adjusted life expectancy (expressed in quality-adjusted life years, QALYs), direct costs and, where required, incremental cost-effectiveness ratios (ICERs), which describe the cost per additional unit of effectiveness gained for the intervention versus the comparator. Projected long-term outcomes have been validated against real-life data in 2004 and more recently in 2014 [8, 9].
The analysis was consistent with previously published cost-effectiveness analyses in the UK setting [10, 11]. A lifetime time horizon was used, as recommended in modelling guidelines for the UK released by NICE, to ensure that all differences in long-term complications (and their impact on costs and quality of life) and mortality with once-weekly semaglutide 1 mg and empagliflozin 25 mg were captured [12]. Projected cost and clinical outcomes were discounted at 3.5% annually, in line with modelling guidelines for the UK [12]. Base case and scenario analyses were performed using a second-order Monte Carlo approach, with baseline cohort characteristics, treatment effects, costs of complications, utilities and transition probabilities relating to myocardial infarction, stroke, congestive heart failure and angina sampled in each model iteration.
Clinical Data
Baseline cohort characteristics were based on pooled data from SUSTAIN 2, SUSTAIN 3, SUSTAIN 8 and PIONEER 2, as these studies informed the meta-analysis. The mean (standard deviation, SD) age of the cohort was 56 (10.3) years, with mean duration of diabetes of 7 (5.9) years, mean HbA1c of 8.2 (1.0)% (66 [11] mmol/mol) and mean BMI of 32.8 (6.7) kg/m2. Approximately 4%, 2% and 1% of patients had a history of myocardial infarction, angina and peripheral vascular disease, respectively. Few patients had a history of renal complications, with less than 1% of patients with a history of microalbuminuria. Of the ophthalmic complications, 8% of patients had a history of background diabetic retinopathy, while proliferative diabetes retinopathy, cataract and severe vision loss were rare. Alcohol and tobacco consumption were assumed to be the same as the general UK population, as these were not collected in the clinical trials [13, 14]. Changes in physiological parameters with once-weekly semaglutide 1 mg and empagliflozin 25 mg were based on the outcomes calculated in the meta-regression based on individual patient data (Table 1) [5]. Once-weekly semaglutide 1 mg was associated with significantly greater improvements in HbA1c, total cholesterol, LDL cholesterol, triglycerides and BMI compared with empagliflozin 25 mg.
Long-term parameter progression and treatment intensification was based on a clinically realistic approach. Following application of the treatment effects in the first year of the analysis, HbA1c was assumed to increase based on the UKPDS progression equation. This resulted in HbA1c increasing in both arms of the analysis, with the difference between the treatment arms gradually diminished. When HbA1c exceeded 7.5% (58 mmol/mol) (the NICE threshold for treatment intensification [15]) patients discontinued once-weekly semaglutide 1 mg or empagliflozin 25 mg and initiated treatment with basal insulin (assumed to be generic insulin glargine [insulin Abasaglar®]). At this stage, a reduction in HbA1c based on an insulin-naïve population derived from the “Core” multivariate equations estimated by Willis et al. was applied [16]. This approach was chosen to reflect common clinical practice where, because of the progressive nature of type 2 diabetes, glycaemic control cannot be maintained indefinitely and the addition of further medications, such as basal insulin, is required [17]. Reductions in BMI were assumed to persist while patients received once-weekly semaglutide 1 mg or empagliflozin 25 mg, with BMI returning to baseline when basal insulin was initiated (thereby abolishing the difference). In both arms, changes in blood pressure and serum lipids over the long-term were based on the natural progression algorithms built into the IQVIA CORE Diabetes Model, based on the UKPDS and Framingham data, respectively.
Costs
All costs were accounted from a UK healthcare payer perspective in 2019 pounds sterling (GBP). Direct costs captured included pharmacy costs, costs associated with diabetes-related complications and patient management costs. Unit costs of diabetes medications were taken from the Monthly Index of Medical Specialities (MIMS) database, and were used to calculate annual pharmacy costs with once-weekly semaglutide 1 mg, empagliflozin 25 mg and basal insulin (assumed 40 IU based on the defined daily dose) [18, 19]. Costs of complications and patient management were consistent with previously published cost-effectiveness analyses for the UK setting, with costs taken from published sources and inflated to 2019 values where appropriate (Table 2) [10, 20].
Utilities
As diabetes progresses, patients develop complications that influence their overall health-related quality of life. It was therefore important to evaluate both mortality and morbidity, and address the utility levels associated with each of the complications modelled. Utilities associated with each diabetes-related complication (Table 3) were taken from a 2014 review by Beaudet et al., with hypoglycaemia disutilities coming from Evans et al. 2013 (published after the literature searches by Beaudet et al. had been completed) [21, 22]. Beaudet et al. reviewed the methods of the identified publications to ensure that they met the criteria of the NICE reference case.
Scenario Analyses
Projection of outcomes over patient lifetimes is associated with uncertainty, and therefore a series of scenario analyses were performed to assess the robustness of the model results. The base case analysis used a 50-year time horizon, and the impact of shortening the time horizon of the analysis was examined by running analyses over 20- and 10-year time horizons. In the base case analysis, discount rates of 3.5% per annum for future clinical and cost outcomes were applied, with a scenario analysis conducted applying 0% discount rates. An analysis was conducted with only the statistically significant differences between the once-weekly semaglutide 1 mg and empagliflozin 25 mg arms applied, whereas the base case analysis applied all treatment effects irrespective of statistical significance.
The base case analysis applied a disutility per BMI unit above 25 kg/m2 of − 0.0061, and a scenario analysis was conducted using an alternative value of − 0.01 to assess the impact of using an alternative source to inform this model input [23]. This larger disutility gives greater impact to weight changes compared with the conservative disutility used in the base case analysis. To assess the impact of the disutilities applied following hypoglycaemic events, a scenario was prepared with alternative disutilities for severe and non-severe hypoglycaemic events applied, as reported by Currie et al. (− 0.0118 per severe hypoglycaemic event and − 0.0035 per non-severe hypoglycaemic event) [24].
The base case analysis used the UKPDS 68 risk equations, but in February 2014, an update to the IQVIA CORE Diabetes Model was released, incorporating data from the UKPDS 82, with these risk equations applied in a scenario analysis. Whilst a validation study of the revised model has been published, the model proprietors suggest that the update is used in a scenario analysis, with the previous version being used in the base case [9]. The base case analysis assumed that HbA1c increased based on the UKPDS progression equation for the duration of the analysis in both arms. An alternative was explored with HbA1c increasing by 0.14% (1.5 mmol/mol) per year in both arms of the analysis while patients received once-weekly semaglutide 1 mg or empagliflozin 25 mg, based on the metformin arm of the ADOPT study [25]. When patients initiated basal insulin, HbA1c followed the UKPDS progression equation, as in the base case.
In the base case analysis, BMI returned to baseline on treatment intensification, and an alternative was explored with BMI returning to baseline followed by a further increase based on the Willis et al. equations [16]. For simplicity, the base case analysis include only one intensification step, and a scenario analysis was conducted with a second intensification to basal bolus insulin when HbA1c exceeded 7.5% (58 mmol/mol) for the second time. A reduction in HbA1c and an increase in BMI were applied, based on the Willis et al. [16] equations for “insulin experienced” patients. Instead of the treatment switching approach based on an HbA1c threshold applied in the base case analysis, an alternative was explored with treatment switching after 3 years in both arms. In this analysis, the difference in HbA1c was held constant while patients received once-weekly semaglutide 1 mg or empagliflozin 25 mg, with HbA1c brought to 7% (32 mmol/mol) on treatment intensification to abolish the difference. This approach is consistent with previously published cost-effectiveness analyses of once-weekly semaglutide [26].
Generic basal insulin glargine was used as the intensification treatment in the base case analysis, as it is the most commonly used generic analogue insulin. Scenario analyses were performed with the costs of insulin Semglee® (the lowest cost analogue insulin available in the UK), and insulin Lantus® (the most commonly used branded insulin analogue) applied to evaluate the impact of intensification with less or more costly basal insulins.
The base case analysis was performed using a second-order Monte Carlo approach, with sampling around inputs to capture both first- and second-order uncertainty. A scenario analysis was performed using a first-order Monte Carlo approach, with no sampling around baseline characteristics, treatment effects, costs of complications utilities or transition probabilities.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Base Case Analysis
Once-weekly semaglutide 1 mg was associated with discounted life expectancy and quality-adjusted life expectancy of 13.59 years and 7.28 QALYs, respectively, compared with 13.47 years and 7.05 QALYs, respectively, with empagliflozin 25 mg (Table 4). The projected improvements of 0.12 years and 0.23 QALYs resulted from a reduced cumulative incidence and delayed time to onset of diabetes-related complications with once-weekly semaglutide 1 mg compared with empagliflozin 25 mg. This was driven by the greater reductions in HbA1c and BMI, and improvements in serum lipids identified in the indirect comparison (Table 1). Additionally, the improved glycaemic control with once-weekly semaglutide 1 mg led to delayed intensification with insulin compared by 1 year with empagliflozin 25 mg (mean times to intensification of 3 years and 2 years with once-weekly semaglutide 1 mg and empagliflozin 25 mg, respectively). This resulted in a delay of the hypoglycaemic events and weight gain associated with insulin therapy, driving further improvements in life expectancy and quality-adjusted life expectancy.
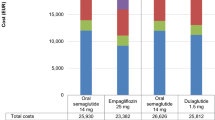
Over patient lifetimes, once-weekly semaglutide 1 mg was associated with a projected cost of GBP 27,144 compared with GBP 26,127 with empagliflozin 25 mg (Fig. 1). The increase in costs with once-weekly semaglutide 1 mg was driven by the higher acquisition cost of once-weekly semaglutide and by the increased duration of treatment by an average of 1 year with once-weekly semaglutide 1 mg compared with empagliflozin 25 mg due to later intensification of therapy as a result of improved glycaemic control. However, cost savings due to avoided diabetes-related complications with once-weekly semaglutide 1 mg partially offset the increased treatment costs. Mean per-patient cost savings of GBP 222 and GBP 102 due to avoided cardiovascular disease and renal disease, respectively, made the most significant contributions, but cost savings were identified in all categories of complications modelled.
Direct costs over patient lifetimes. GBP 2019 pounds sterling, QALY quality-adjusted life year. Treatment costs captured the costs of diabetes medications and consumables; management costs included the costs of concomitant non-diabetes medications and screening; costs of cardiovascular complications included the costs of myocardial infarction, angina, congestive heart failure, stroke and peripheral vascular disease; costs of renal complications included the costs of dialysis and transplant, costs of ulcer, amputation and neuropathy complications included the costs of ulcer, gangrene, amputation, prosthesis and neuropathy; costs of ophthalmic complications captured the costs of laser treatment, cataract surgery and blindness; costs of hypoglycaemia included the costs of severe and non-severe hypoglycaemic events
When clinical and cost outcomes were combined to assess cost-effectiveness, once-weekly semaglutide 1 mg was associated with an ICER of GBP 4439 per QALY gained versus empagliflozin 25 mg over patient lifetimes. In 76.5% of model iterations once-weekly semaglutide 1 mg was associated with improved outcomes and increased costs compared with empagliflozin 25 mg, and in a further 18.6% of model iterations once-weekly semaglutide 1 mg was associated with improved outcomes and cost savings (Fig. 2). At a willingness to pay threshold of GBP 20,000 per QALY gained, the commonly quoted willingness to pay threshold used by NICE in the UK setting, there was a 91% probability that once-weekly semaglutide 1 mg was considered cost-effective (Fig. 3).
Scenario Analysis Results
Semaglutide 1 mg remained cost effective versus empagliflozin 25 mg when alternative model inputs and assumptions were applied, with all calculated ICERs remaining well below GBP 20,000 per QALY gained (Table 5). Application of shorter time horizons led to higher ICERs as the long-term benefits of once-weekly semaglutide 1 mg were not fully captured, as the improvements in physiological parameters associated with once-weekly semaglutide 1 mg reduced the risk of diabetes-related complications over patient lifetimes. Removing discounting resulted in increased cost-effectiveness of once-weekly semaglutide 1 mg versus empagliflozin 25 mg, further reflecting the long-term benefits associated with once-weekly semaglutide 1 mg.
Applying only treatment effects that were significantly different between the treatment arms resulted in only small changes in projected quality-adjusted life expectancy and costs, with the ICER remaining similar to the base case analysis.
Applying a disutility which increased the impact of weight loss on quality-adjusted life expectancy resulted in a lower ICER than in the base case analysis, due to the greater reduction in BMI with once-weekly semaglutide 1 mg compared with empagliflozin 25 mg. Use of disutilities that reduced the impact of hypoglycaemic events on quality of life resulted in increased quality-adjusted life expectancy in both treatment arms, with a small reduction in clinical benefits with once-weekly semaglutide 1 mg as the increased frequency of hypoglycaemia in the empagliflozin 25 mg arm due to earlier intensification had a smaller impact on long-term outcomes.
To assess the uncertainty around HbA1c increases over time, an analysis was performed in which it was assumed that HbA1c increased by 0.14% (1.5 mmol/mol) per year in both arms rather than based on the UKPDS progression equation. In this analysis, differences in HbA1c were maintained for longer than in the base case, resulting in a greater improvement in quality-adjusted life expectancy with once-weekly semaglutide 1 mg versus empagliflozin 25 mg. This resulted in a lower ICER than in the base case analysis.
Use of the UKPDS 82 risk equations resulted in increased quality-adjusted life expectancy in both arms, but the clinical benefit with once-weekly semaglutide 1 mg was smaller than in the base case. Costs were slightly higher than in the base case in both arms, with the cost increase associated with once-weekly semaglutide slightly reduced. Overall, the ICER remained similar to the base case analysis.
Increasing BMI to above baseline levels in both arms on intensification with basal insulin resulted in reduced quality-adjusted life expectancy in both arms, but incremental outcomes and the calculated ICERs showed only small changes. Inclusion of a second intensification resulted in reduced quality-adjusted life expectancy in both arms due to increased BMI and hypoglycaemic event rates associated with basal-bolus insulin. Costs were also increased in both arms, due to the additional cost of bolus insulin. The additional delay in the second intensification with once-weekly semaglutide 1 mg compared with empagliflozin 25 mg resulted in greater clinical benefits, a smaller increase in costs and a lower ICER than in the base case.
In the analysis where patients received once-weekly semaglutide 1 mg or empagliflozin 25 mg for a fixed 3-year time period before treatment intensification, with HbA1c, BMI and pharmacy cost differences maintained only while patients received initial treatments, clinical benefits with once-weekly semaglutide 1 mg were smaller than in the base case analysis with the difference in costs remaining similar. This resulted in an ICER of GBP 13,393 per QALY gained. This was the highest ICER identified across the scenario analyses, though remaining well below the GBP 20,000 per QALY gained willingness to pay threshold.
Intensifying therapy with more or less costly basal insulin formulations available in the UK had only a small impact on cost outcomes, with ICERs remaining similar to the base case analysis. Similarly, in the analysis in which sampling of patient characteristics and treatment effects was turned off, projected outcomes remained similar to the base case analysis.
Discussion
A previously published meta-analysis found that once-weekly semaglutide 1 mg was associated with greater improvements in HbA1c, lipids and BMI versus empagliflozin 25 mg [5]. The present analysis projected outcomes over patient lifetimes based on the results of this indirect comparison, and found that these changes in risk factors resulted in reduced cumulative incidence and delayed time to onset of diabetes-related complications, increased life expectancy and increased quality-adjusted life expectancy with once-weekly semaglutide 1 mg compared with empagliflozin 25 mg. While once-weekly semaglutide 1 mg was associated with increased pharmacy costs compared with empagliflozin 25 mg, this was partially offset by reduced costs of diabetes-related complications. The projected ICER of GBP 4439 per QALY gained falls well below the willingness to pay threshold of GBP 20,000 per QALY gained. Once-weekly semaglutide 1 mg was projected to be a cost-effective treatment from a healthcare payer perspective compared with empagliflozin 25 mg for the treatment of patients with type 2 diabetes in the UK setting. Scenario analyses confirmed that the conclusions of the analysis were robust to realistic changes in model inputs and assumptions.
Projections of long-term outcomes are dependent on the model inputs, and therefore the strengths and weaknesses of the previously published meta-analysis must be considered to put the results of the present analysis into context. Use of individual patient data allowed potential prognostic factors and effect modifiers to be adjusted, which is not possible when aggregated data is used. Furthermore, the use of individual patient data allowed a wider range of outcomes to be assessed (such as lipid parameters), that would not be possible based on trial-level data as these are infrequently reported in the peer-reviewed literature, giving a more accurate reflection of the differences in risk factors for diabetes-related complications. All of the included trials were of a similar design and applied similar inclusion/exclusion criteria, with minimal heterogeneity between the trials. However, there were some differences in trials design, such as blinding and the study duration (52 versus 56 weeks). The impact of study duration on results was explored within the complementary analysis in the published meta-analysis, which confirmed no significant impact. A limitation of the approach is that no common comparator was included, and this can limit the adjustment. Unanchored indirect comparisons assume that all potential prognostic factors and effect modifiers are identified, and this is difficult to verify and therefore was assumed to be the case within the published meta-analysis. It should also be noted that data from randomized controlled trials were used to assess the cost-effectiveness of interventions in the real world, and the therefore there is an implicit assumption that efficacy is similar in randomized controlled trials and routine clinical practice. How well the cohort modelled in the present analysis represent patients with type 2 diabetes in the UK is hard to assess, but it should be noted that the cohort characteristics from the meta-analysis are similar to those used in the economic modelling conducted to support the NICE NG28 guidelines, based on The Health Improvement Network (THIN) [27].
The meta-regression used to inform the present cost-effectiveness analysis used individual patient data to compare once-weekly semaglutide 1 mg with empagliflozin 25 mg, but a network meta-analysis using trial-level data (i.e. based on mean outcomes across the included trial participants) comparing once-weekly semaglutide 0.5 mg and 1 mg with SGLT2 inhibitors, including empagliflozin 25 mg, has also been published [28]. This network meta-analysis reported estimated treatment differences for once-weekly semaglutide 1 mg versus empagliflozin 25 mg, with estimated treatment difference in change from baseline in HbA1c of − 0.80% (95% CI − 1.04 to − 0.58%) (− 8.7 mmol/mol [95% CI − 11.4 to − 6.3 mmol/mol]), estimated treatment difference in change from baseline in weight of − 2.05 kg (95% CI − 2.94 to − 1.15 kg) and estimated treatment difference in change from baseline in systolic blood pressure of − 2.47 mmHg (95% CI − 5.79 to 0.83 mmHg). These results are similar to the results of the meta-regression based on individual patient data, with both showing significantly greater reductions in HbA1c and weight with once-weekly semaglutide 1 mg versus empagliflozin 25 mg. The similarity in these outcomes indicates that input values used within the present analysis are robust. As a result of the similarities between the results of the two indirect comparisons, conducting a scenario analysis using the network meta-analysis data was not considered necessary.
Previous to the present analysis, two studies have assessed the cost-effectiveness of once-weekly semaglutide from the perspective of a UK healthcare payer. When once-weekly semaglutide 0.5 mg and 1 mg were compared with dulaglutide 1.5 mg based on the SUSTAIN 7 randomized controlled trial, both doses of once-weekly semaglutide were associated with improved clinical outcomes and cost savings compared with dulaglutide 1.5 mg [26]. Therefore both doses of once-weekly semaglutide were considered dominant. More recently, once-weekly semaglutide 1 mg was compared with liraglutide 1.2 mg based on the SUSTAIN 10 randomized controlled trial [10]. This analysis also found that once-weekly semaglutide 1 mg was associated with improved clinical outcomes and cost savings compared with liraglutide 1.2 mg, and therefore was found to be dominant. Previous analyses in the UK have compared once-weekly semaglutide with other GLP-1 receptor agonists, and this study is the first to compare with an SGLT2 inhibitor. The present analysis contributes to the growing body of evidence that once-weekly semaglutide is a cost-effective treatment option in the UK for patients with type 2 diabetes mellitus.
The approach of the present analysis was aligned with clinical practice and previously published cost-effectiveness analyses. The analysis aimed to take a single clinical approach, with glycaemic control declining over time, with subsequent addition of insulin therapy and a consequent reduction in HbA1c accompanied by an increase in BMI and risk of hypoglycaemia. This approach has been used in peer-reviewed cost-effectiveness analyses, and in the NICE multiple technology appraisal of SGLT2 inhibitors [10, 11, 29]. The intention behind using this approach was to ensure that the analysis reflects clinical practice and is therefore relevant to healthcare decision makers.
However, the analysis only captured only one treatment pathway, with intensification from GLP-1 receptor agonists or SGLT2 inhibitor to basal insulin, with the previous treatment discontinued. It should be noted that alternative treatment pathways may be used in clinical practice, such as addition of an SGLT2 inhibitor to a GLP-1 receptor agonist, addition of a GLP-1 receptor agonist to an SGLT2 inhibitor, or continuation of previous therapies alongside basal insulin [3]. Currently there is a lack of clinical data to allow modelling of more complex treatment pathways, and this is an area for future study as the clinical evidence base expands. The present analysis used a simple treatment pathway in order to answer the research question on the relative cost-effectiveness of the two interventions for the treatment of patients with type 2 diabetes with inadequate glycaemic control on metformin monotherapy, with both interventions used until glycaemic control could no longer be maintained.
The analysis also compared only two interventions (once-weekly semaglutide and empagliflozin) at one point in the diabetes-treatment algorithm (as part of dual therapy for treatment of patients with inadequate glycaemic control on metformin monotherapy). In order to have a full overview of the cost-effectiveness of interventions for the treatment of diabetes, cost-effectiveness should be assessed at each stage of the treatment algorithm versus all relevant comparators. This includes versus oral medications as dual therapy and triple therapy, and versus insulin as triple and quadruple therapy. Such analyses were beyond the scope of the present study, but should be conducted in the future to provide all relevant data to healthcare decision makers.
A limitation of the analysis is that data from cardiovascular risk trials were not included in the long-term projections. Both once-weekly semaglutide and empagliflozin have been shown to reduce the risk of major adverse cardiovascular events compared with standard care in the SUSTAIN 6 and EMPA-REG OUTCOME trials, respectively [30, 31]. However, data from cardiovascular outcomes trials (CVOTs) have not yet been incorporated into risk equations for projecting long-term outcomes in people with type 2 diabetes. This is challenging, as CVOTs generally enrol participants at high risk of experiencing cardiovascular events, and how benefits would translate to lower-risk populations, such as those participating in the trials included in the meta-regression, is unclear. Furthermore, the separation of the impact of changes in conventional risk factors (such as HbA1c, blood pressure, serum lipids and body weight) associated with treatments from the direct impact on cardiovascular risk associated with the treatments themselves is not possible based on data currently in the public domain. Early analyses suggest that capturing cardiovascular risk can have an important impact on the results of long-term analyses, and therefore including this in revised risk equations represents a key goal for the future [32]. Cardiovascular events are a key driver of the clinical and economic burden of type 2 diabetes, and therefore accurate prediction of risk is crucial in order to make informed decisions, with early control of risk factors and therefore avoidance of events particularly important from a healthcare payer perspective.
A limitation common to the majority of cost-effectiveness of type 2 diabetes is the projection of outcomes over patient lifetimes in a real-world setting based on short-term clinical trial data. This approach is associated with inherent uncertainty, due to the uncertainty around how risk factors for diabetes-related complications change over time and how well risk equations based on historic data predict outcomes for modern patients. However, projection of long-term outcomes is recommended in modelling guidelines and remains the best available option to inform decision-making in the absence of long-term clinical trial data. In the present analysis, the impact of this uncertainty has been mitigated as far as possible by using a model that has been extensively published and validated, and by conducting extensive scenario analyses using different inputs and assumptions and by using clinical inputs from a robust data source [5, 8, 9].
Conclusions
Once-weekly semaglutide 1 mg was projected to be a cost-effective treatment from a healthcare payer perspective compared with empagliflozin 25 mg for the treatment of patients with type 2 diabetes mellitus with inadequate glycaemic control on metformin monotherapy in the UK setting based on a willingness to pay threshold of GBP 20,000 per QALY gained as used by NICE. The results of the present analysis should be considered during clinical decision-making and when NICE guidelines on diabetes management in the UK are updated.
References
International Diabetes Foundation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019. http://www.diabetesatlas.org. Accessed Sep 2020.
Diabetes UK. Facts and figures. 2019. https://www.diabetes.org.uk/resources-s3/2019-02/1362B_Facts%20and%20stats%20Update%20Jan%202019_LOW%20RES_EXTERNAL.pdf. Accessed Oct 2020.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221–8.
Lingvay I, Capehorn MS, Catarig AM, et al. Efficacy of once-weekly semaglutide vs empagliflozin added to metformin in type 2 diabetes: patient-level meta-analysis. J Clin Endocrinol Metab. 2020. https://doi.org/10.1210/clinem/dgaa577.
Phillippo DM, Ades AE, Dias S, et al. NICE DSU Technical Support Document 18: methods for population-adjusted indirect comparisons in submission to NICE. 2016. http://www.nicedsu.org.uk. Accessed Sep 2020.
Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl_1):S5-26.
Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27-40.
McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17(6):714–24.
Johansen P, Chubb B, Hunt B, Malkin SJP, Sandberg A, Capehorn M. Evaluating the long-term cost-effectiveness of once-weekly semaglutide versus once-daily liraglutide for the treatment of type 2 diabetes in the UK. Adv Ther. 2020;37(5):2427–41.
Bain SC, Hansen BB, Malkin SJP, et al. Oral semaglutide versus empagliflozin, sitagliptin and liraglutide in the UK: long-term cost-effectiveness analyses based on the PIONEER clinical trial programme. Diabetes Ther. 2020;11(1):259–77.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed Sep 2020.
Office for National Statistics. Adult smoking habits in Great Britain. 2017. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/datasets/adultsmokinghabitsingreatbritain. Accessed Sep 2020.
World Health Organization. Global alcohol report. 2014. http://www.who.int/substance_abuse/publications/global_alcohol_report/profiles/gbr.pdf. Accessed Sep 2020.
National Institute for Health and Care Excellence. Type 2 diabetes in adults: management [NG28]. Updated August 2019. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#blood-glucose-management-2. Accessed Sep 2020.
Willis M, Asseburg C, Nilsson A, Johnsson K, Kartman B. Multivariate prediction equations for HbA1c lowering, weight change, and hypoglycemic events associated with insulin rescue medication in type 2 diabetes mellitus: informing economic modeling. Value Health. 2017;20:357–71.
Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–6.
Monthly Index of Medical Specialities (MIMS). 2020. https://www.mims.co.uk/. Accessed Sep 2020.
WHO Collaborating Centre for Drug Statistics MethodologyATC/DDD index. https://www.whocc.no/atc_ddd_index/?code=A10AE&showdescription=no. Accessed Sep 2020.
Curtis L, Burns A. Unit costs of health and social care 2018. Personal social services research unit. Canterbury: University of Kent; 2018.
Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–70.
Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11(1):90.
Lee AJ, Morgan CL, Morrissey M, Wittrup-Jensen KU, Kennedy-Martin T, Currie CJ. Evaluation of the association between the EQ-5D (health-related utility) and body mass index (obesity) in hospital-treated people with type 1 diabetes, type 2 diabetes and with no diagnosed diabetes. Diabet Med. 2005;22(11):1482–6.
Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–34.
Viberti G, Kahn SE, Greene DA, et al. A Diabetes Outcome Progression Trial (ADOPT): an international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care. 2002;25(10):1737–43.
Viljoen A, Hoxer CS, Johansen P, Malkin S, Hunt B, Bain SC. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21(3):611–21.
National Institute for Health and Care Excellence. Type 2 diabetes in adults: management (NG28): Appendix F: full health economics report. https://www.nice.org.uk/guidance/ng28/documents/type-2-diabetes-appendix-f2. Accessed Dec 2020.
Sharma R, Wilkinson L, Vrazic H, et al. Comparative efficacy of once-weekly semaglutide and SGLT-2 inhibitors in type 2 diabetic patients inadequately controlled with metformin monotherapy: a systematic literature review and network meta-analysis. Curr Med Res Opin. 2018;34(9):1595–603.
National Institute for Health and Care Excellence. Canagliflozin, dapagliflozin and empagliflozin monotherapy for treating type 2 diabetes. Assessment report. Commercial in confidence stripped version for consultation 2015. https://www.nice.org.uk/guidance/ta390/documents/assessment-report. Accessed Sep 2020.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Evans M, Johansen P, Vrazic H, Pitcher AB, Falla E. The importance of incorporating cardio-protective effects of once-weekly semaglutide in estimates of health benefits for patients with type 2 diabetes. Diabetologia. 2018;61:427–8.
Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabetes Med. 2015;32(4):459–66.
Danese M, Gleeson M, Kutikova L, et al. Costs of cardiovascular (CV) events in the United Kingdom (UK) using real-world data. Value Health. 2015;18:A335–766.
NHS England. Reference costs. 2018. https://improvement.nhs.uk/resources/reference-costs/#rc1718. Accessed Sep 2020.
Kent S, Schlackow I, Lozano-Kuhne J, et al. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease? BMC Nephrol. 2015;16:65.
Chubb B, Tikkanen C. The cost of non-severe hypoglycaemia in Europe. Value Health. 2015;18(7):A611.
Hammer M, Lammert M, Mejias SM, Kern W, Frier BM. Costs of managing severe hypoglycaemia in three European countries. J Med Econ. 2009;12(4):281–90.
Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with Promogran in four European countries. J Wound Care. 2002;11(2):70–4.
Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002;22(4):340–9.
Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14(3):217–30.
Wasserfallen JB, Halabi G, Saudan P, et al. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2004;19(6):1594–9.
Kiberd BA, Jindal KK. Screening to prevent renal failure in insulin dependent diabetic patients: an economic evaluation. BMJ. 1995;311(7020):1595–9.
Fenwick EK, Xie J, Ratcliffe J, et al. The impact of diabetic retinopathy and diabetic macular edema on health-related quality of life in type 1 and type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53(2):677–84.
Lee WJ, Song KH, Noh JH, Choi YJ, Jo MW. Health-related quality of life using the EuroQol 5D questionnaire in Korean patients with type 2 diabetes. J Korean Med Sci. 2012;27(3):255–60.
Acknowledgements
Funding
This study and corresponding journal’s rapid service fee were supported by funding from Novo Nordisk A/S, Søborg, Denmark. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Matthew Capehorn has received honoraria from advisory board meetings for BI/Lilly Alliance, Janssen, MSK, Novo Nordisk; payments for speaker meetings from Abbot, BI/Lilly Alliance, Novo Nordisk, Sanofi-Aventis; travel and/or accommodation expenses to attend educational meetings from BI/Lilly, Lighterlife, Novo Nordisk; and is a partner and Clinical Manager of the Rotherham Institute for Obesity (RIO), Director of RIO Weight Management Ltd., Medical Director at Lighterlife (paid), board member of the Association of the Study for Obesity (ASO) (unpaid), and an Expert Advisor to the National Institute for Health and Care Excellence (unpaid); RIO has received research funding in the past or currently from Abbot, Bayer, BI/Lilly Alliance, Cambridge Weight Plan, GSK, Janssen, Leo Pharma, Lighterlife, Merck, Novartis, Novo Nordisk. Nino Hallén and James Baker-Knight are employees of Novo Nordisk A/S. Barnaby Hunt is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis. James Baker-Knight and Divina Glah have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Capehorn, M., Hallén, N., Baker-Knight, J. et al. Evaluating the Cost-Effectiveness of Once-Weekly Semaglutide 1 mg Versus Empagliflozin 25 mg for Treatment of Patients with Type 2 Diabetes in the UK Setting. Diabetes Ther 12, 537–555 (2021). https://doi.org/10.1007/s13300-020-00989-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00989-6