Abstract
Introduction
Sodium-glucose cotransporter 2 (SGLT2) inhibitors promote urinary glucose excretion. However, the differences in the effects of various SGLT2 inhibitors are unknown. We used flash glucose monitoring (FGM) to identify the differences between tofogliflozin and ipragliflozin in terms of efficacy in reducing glycemic variability and mitigate hypoglycemia risk.
Methods
In this crossover study, 24 patients with type-2 diabetes mellitus (T2DM) receiving insulin glargine U300 therapy were randomly allocated to tofogliflozin and ipragliflozin or ipragliflozin and tofogliflozin group. Glycemic variability and hypoglycemia were compared using to the 3-day FGM data per treatment period.
Results
Glucose level 2 h after each meal was significantly lower with tofogliflozin administration than with ipragliflozin administration. Time below the target glucose range after tofogliflozin administration was significantly lower than that after ipragliflozin administration (2.1% ± 4.4% vs. 8.7% ± 11.7%). The 24-h standard deviation of glucose level, mean amplitude of glycemic excursion, and mean percent time with nocturnal hypoglycemia after tofogliflozin administration were significantly lower than those after ipragliflozin administration.
Conclusions
Tofogliflozin was more effective and safer than ipragliflozin in reducing glycemic variability and mitigating hypoglycemia risk in patients with T2DM treated with insulin glargine U300.
Trial Registration
This trial was registered at the University Hospital Medical Information Network Clinical Trial Registry (no. UMIN000037158).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Sodium-glucose cotransporter 2 (SGLT2) inhibitors promote urinary glucose excretion. |
The differences in the effects of various SGLT2 inhibitors are unknown. |
Flash glucose monitoring (FGM) was used to identify the differences in efficacy between tofogliflozin and ipragliflozin treatments. |
What was learned from the study? |
Data collected using FGM demonstrated that tofogliflozin was more effective and safer than ipragliflozin in reducing diurnal glycemic variability and lowering the risk of nocturnal hypoglycemia. |
The risk of cardiovascular disease was higher in patients with advanced renal dysfunction; it is unclear whether SGLT2 inhibitors can reduce the risk of cardiovascular diseases in such patients. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13019762.
Introduction
The objectives of diabetes therapy are to delay or prevent the onset of the associated complications and maintain a quality of life of patients comparable to that of healthy individuals [1]. Cardiovascular diseases are a major cause of mortality of patients with diabetes as a comorbidity, and the mortality rate is approximately two times that of patients without diabetes [2]. Therefore, it is important to select treatments that cause minimal glycemic variability and hypoglycemia [3, 4]. An 8-year study in patients with diabetes reported that hypoglycemia (< 70 mg/dl) increased relative to the overall mortality by 1.84 fold [5]. Postprandial glucose level must be suppressed to reduce glycemic variability. The glucose level 2 h after lunch has been reported to be significantly correlated with cardiovascular events and mortality (hazard ratios 1.507 and 1.885, respectively) [6]. Treatment guidelines for type-2 diabetes mellitus (T2DM) recommend the administration of a glucagon-like peptide 1 receptor agonist (GLP-1 RA) and a sodium–glucose cotransporter 2 (SGLT2) inhibitor to patients at a high risk of atherosclerotic cardiovascular diseases (ASCVDs), chronic kidney diseases (CKDs), and heart failure (HF) and those with a history of these disorders. These therapies might prevent the onset and recurrence of the aforementioned conditions [7].
SGLT2 inhibitors are oral hypoglycemic drugs that promote urinary glucose excretion by inhibiting glucose reabsorption in the proximal tubules. Dapagliflozin was the first SGLT2 inhibitor, approved in 2012. At that time, incretin-based therapy was a recommended treatment option for diabetes [8], as dipeptidyl peptidase-4 (DPP-4) inhibitors suppress glycemic variability and reduce hypoglycemia risk [9]. Furthermore, physicians were hesitant to prescribe SGLT2 inhibitors owing to adverse events such as an increased risk of female genital organ infections, urinary tract infections, diabetic ketoacidosis, and bone fractures [10]. SGLT2 inhibitors alleviate several arteriosclerosis risk factors by increasing urinary glucose excretion, promoting body weight and visceral fat mass loss, improving lipid metabolism and uric acid levels, and modulating blood pressure via osmotic diuresis and natriuresis [11,12,13,14]. SGLT2 inhibitors may protect vital organs besides lowering the blood glucose level. Hence, they have been prescribed for patients with diabetes at a high risk of cardiovascular events caused by hypertension and dyslipidemia. In this category of patients with T2DM, adjunct empagliflozin therapy with the standard treatment reduces the incidence of cardiovascular system-related mortality, cardiovascular events, and all-cause mortality [15]. Compared with a placebo, canagliflozin reduced the risk of cardiovascular events in patients with T2DM at a high risk of cardiovascular diseases [16]. Conversely, DPP-4 inhibitors have not been shown to suppress cardiovascular diseases [17,18,19]. On the basis of these findings, SGLT2 inhibitors have been recommended for high-risk T2DM patients with ASCVD. In Japan, six types of SGLT2 inhibitors are approved for T2DM treatment. However, it is unknown whether SGLT2 inhibitors prevent cardiovascular events. Moreover, the effects of SGLT2 inhibitors vary owing to the differences in their pharmacologic properties [20]. Specifically, the pharmacologic characteristics of tofogliflozin and ipragliflozin are different. Drugs with a similar mode of action may have varying efficacy as they may vary in absorption, distribution, metabolism, and excretion [21, 22]. Compared with a placebo, tofogliflozin significantly decreased postprandial glucose level and significantly increased 24-h urinary glucose excretion during a 12-week observation period [23]. Continuous glucose monitoring (CGM) data collected before and 7 days after oral ipragliflozin administration revealed that the drug significantly decreased both preprandial and postprandial glucose levels [24].
The percentage of time in the target glucose range per day (TIR) (70–180 mg/dl) and that below the target glucose range (TBR) (level 1: < 70 mg/dl; level 2: < 54 mg/dl) are the recommended metrics for CGM. The suggested thresholds are ≥ 70% for TIR, ≤ 4% for TBR level 1, and < 1% for TBR level 2 for patients who are not elderly and/or not at a high risk of diabetes but with a significant risk of severe hypoglycemia due to age, diabetes duration, insulin therapy, and relatively higher prevalence of undetected hypoglycemia [25].
It has been reported that tofogliflozin and ipragliflozin effectively control postprandial hyperglycemia [24, 26]. However, only a few studies have directly compared these two agents. Hence, the aim of this study was to determine whether the treatment with tofogliflozin and ipragliflozin and that with ipragliflozin and tofogliflozin differ in terms of their efficacy to limit glycemic variability and hypoglycemia induction risk. For this purpose, we performed FGM of patients with T2DM after their preprandial glucose level at breakfast was titrated with long-acting insulin.
Methods
Study Design and Participants
This single-center, randomized, open-label, parallel-group, crossover study was conducted in patients with T2DM from June 2019 to March 2020 in accordance with the Declaration of Helsinki (1975; revised 2013). The study protocol was approved by the Minami Osaka Hospital Ethics Committee (no. 2018-16) and registered in the University Hospital Medical Information Network Clinical Trial Registry (no. UMIN000037158). All participants were briefed on the study outline before their participation, and they provided written informed consent.
Twenty-four patients with T2DM (14 men and 10 women) were enrolled. They were admitted to Minami Osaka Hospital for glycemic control. Patient selection criteria were as follows: (1) age range of 20–75 years; (2) diagnosis of T2DM ≥ 1 year before screening; (3) basal insulin added to oral agents was administered in the form of insulin glargine U300 for > 3 months before screening; (4) glycated hemoglobin (HbA1c) level was in the range of ≥ 7.0% to < 10.5%; (5) body mass index (BMI) within the range of ≥ 20.0 kg/m2 to < 45.0 kg/m2; (6) estimated glomerular filtration rate (eGFR) of ≤ 45 ml/min/1.73 m2; (7) no SGLT2 inhibitor was administered for > 6 months before screening. The exclusion criteria were as follows: (1) severe ketosis, severe hypoglycemia, diabetic coma or precoma, and subjective urinary tract or genital organ infection within 6 weeks before screening; (2) malignant tumor, history of malignant tumor, renal vascular obstructive disease, nephrectomy, renal transplantation, dysuria, anuria, oliguria, or urinary retention; (3) diabetic proliferative retinopathy, except for therapeutically stabilized patients; (4) severe gastrointestinal tract disorder or history of surgical intervention for gastrointestinal tract disorder within 2 weeks before the study; (5) acute coronary syndrome or cerebrovascular disorder within 3 months before the study; (6) putative or confirmed pregnancy or lactation, severe infections or systemic corticosteroid administration; (8) severe liver dysfunction [aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels > 100 U/l]; (9) history of SGLT2 inhibitor allergy.
Figure 1 shows the protocol of this study. Twenty-four participants were randomly assigned to the tofogliflozin and ipragliflozin group or to the ipragliflozin and tofogliflozin group at a ratio of 1:1 by block randomization using a random number table. During the study period, self-monitoring of blood glucose (SMBG) was performed four times daily before meals and at bedtime. In the tofogliflozin and ipragliflozin group, the participants received 20 mg tofogliflozin once after breakfast and then insulin glargine U300, which has a small daily fluctuation and an excellent blood glucose-stabilizing effect [27], was administered once daily before breakfast to achieve the target preprandial glucose levels at breakfast, that is, > 80 and < 110 mg/dl. If hypoglycemic symptoms appeared in participants with a glucose level above the target preprandial glucose level, the insulin dose was lowered at the discretion of the doctor in charge. The insulin titration period was ≥ 7 days to eliminate the effect of glucose toxicity. All participants wore a Freestyle Libre Pro™ flash glucose monitoring (FGM) system (Abbott Diabetes Care, Alameda, CA, USA) when the average 3-day glycemic variability of the preprandial glucose level at breakfast, as determined by SMBG, was within 10%. Blood was sampled using the FGM system on days 4 and 11, and serum albumin level was measured at 0600 and 2100 h. Urine glucose excretion was measured on days 4 and 11 between 0800 and 2200 h and again between 2200 and 0800 h on the next morning. The interstitial glucose levels determined by FGM from days 2 to 14 were found to be accurate compared with capillary blood glucose levels [28]. Therefore, data of tofogliflozin were collected from days 4 to 6 of wearing the FGM system. On day 7 of wearing the FGM system, tofogliflozin was replaced with 50 mg ipragliflozin administered once after breakfast. To eliminate any residual tofogliflozin effect, data on the ipragliflozin effect were collected from days 11 to 13 of wearing the FGM system, and the patients were observed for 4 days after switching to ipragliflozin.
For the ipragliflozin and tofogliflozin group, the participants received 50 mg ipragliflozin once after breakfast before tofogliflozin administration and the aforementioned regimen was maintained. All antihyperglycemic drugs that were administered to the patients before participation in the study were continued, and during the FGM period, the doses of insulin glargine U300 and all antihyperglycemic drugs except the SGLT2 inhibitors were not changed.
Food intake by the participants in the hospital was 25–30 kcal/ideal body weight/day. The nutrient ratio was 60% carbohydrate:17% protein:23% lipid, and the calorie allocation ratio was 30% breakfast:35% lunch:35% supper. The participants performed moderate aerobic exercise for 30 min/day.
Outcome Measures
The efficacy and safety of the primary and secondary endpoints were evaluated based on the FGM data of 3 consecutive days. The primary efficacy endpoint was the glucose level 2 h after each meal [29]. The primary safety endpoint was TBR level 1 [25]. The secondary endpoints were TIR, time above the TAR (> 180 mg/dl) [25], 24-h standard deviation (SD) of glycemic variability [30], 24-h M-value (target glucose level = 100 mg/dl) [31], mean amplitude of glycemic excursion (MAGE) [31], mean of daily difference (MODD) for a 24-h period (average of the difference in FGM data for days 1–2 and days 2–3 over 3 consecutive days) [31], 24-h mean glucose levels, 0000–0600 h mean glucose levels, preprandial glucose levels at each meal, TBR level 2 [25], nocturnal TBR (< 70 mg/dl), area under the glucose curve (AUC) for diurnal glycemic variability [32], AUC for glycemic variability at 0800–2200 h, AUC for glycemic variability at 2200–0800 h, 24-h urinary glucose excretion (UGE) [33], 0800–2200 h UGE, and 2200–0800 h UGE.
Statistical Analysis
Data are presented as mean ± SD unless otherwise specified. Differences between tofogliflozin and ipragliflozin were evaluated using Student’s t test [34]. A Pearson product-moment correlation test was used to determine the correlation coefficient. A two-tailed p < 0.05 indicated significant differences between treatment means. An a priori power analysis was performed using two-tail effect = 0.6, α error = 0.05, and power = 0.8. These parameters indicated that a sample size of 24 was sufficient. Data were analyzed using EZR v. 1.37 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [35].
Results
Participant Characteristics
Table 1 shows the baseline characteristics of the 24 participants randomly assigned to the tofogliflozin and ipragliflozin group and the ipragliflozin and tofogliflozin group. All participants completed the study (Supplementary Fig. S1). Their mean age was 63.4 years, average body mass index (BMI) was 26.6 kg/m2, mean HbA1c level was 8.3%, and average estimated glomerular filtration rate (eGFR) was 70.1 ml/min/1.73 m2. They presented with mild renal dysfunction. All other parameters were similar between the groups. There was no significant difference between the groups in terms of antihyperglycemic drug type and dosage except for those of the SGLT2 inhibitors (all p > 0.05).
Comparison of Tofogliflozin and Ipragliflozin Efficacy and Safety
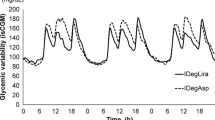
Figure 2 shows the glycemic variability associated with tofogliflozin and ipragliflozin measured by FGM for 3 consecutive days during the treatment period. Postprandial glycemic variability was low for tofogliflozin treatment, and nocturnal glycemic variability was low for ipragliflozin treatment.
Table 2 shows the glycemic variability parameters obtained by FGM. The glucose level 2 h after each meal (breakfast, lunch, and supper) was the primary efficacy endpoint in this study. It was significantly lower in response to tofogliflozin treatment than to ipragliflozin treatment (p = 0.020, 0.040, and 0.014 respectively). The TBR level 1 was the primary safety endpoint. It was significantly lower after tofogliflozin treatment than after ipragliflozin treatment (p < 0.001).
The TIR and TAR after tofogliflozin treatment were significantly higher and lower, respectively, than those after ipragliflozin treatment (p < 0.001 and p = 0.044, respectively). The 24-h SD of glycemic variability and MAGE after tofogliflozin treatment were significantly lower than those after ipragliflozin treatment (p < 0.001). However, there was no significant difference between tofogliflozin and ipragliflozin treatments in terms of the 24-h M-value (target glucose level = 100 mg/dl) or MODD (p > 0.05). The 24-h mean glucose level did not significantly differ between the two treatment groups. Nevertheless, the mean glucose level at 0000–0600 h after ipragliflozin treatment was significantly lower than that after tofogliflozin treatment (p = 0.021). There were no significant differences between the treatments in terms of the preprandial glucose level of each meal (p > 0.05). The safety indices TBR level 2 and nocturnal TBR (< 70 mg/dl) after tofogliflozin treatment were significantly lower than those after ipragliflozin treatment (p = 0.001 and p < 0.001, respectively). The efficacy indices AUC for glycemic variability from 0800 to 2200 h and AUC for glycemic variability from 2200 to 0800 h did not significantly differ between the treatments (p > 0.05). There was no significant difference in the 24-h UGE between the treatments but the 0800–2200 h UGE after tofogliflozin treatment was significantly higher than that after ipragliflozin treatment (p > 0.05 and p = 0.005, respectively). Conversely, the 2200–0800 h UGE after ipragliflozin treatment was significantly higher than that after tofogliflozin treatment (p < 0.001).
Correlation Between AUC for Glycemic Variability and UGE in Patients Treated with Tofogliflozin and Ipragliflozin
Here, we evaluated factors affecting glycemic variability. For both tofogliflozin and ipragliflozin treatments, there was a significant negative correlation between the 0800–2200 h AUC for glycemic variability and the 0800–2200 h UGE. In contrast, there was no significant correlation between the 2200–0800 h AUC for glycemic variability and the 2200–0800 h UGE (Fig. 3). The associations between the aforementioned factors and the changes in serum albumin level were investigated as the latter might also influence glycemic variability. For both tofogliflozin and ipragliflozin treatments, no significant correlations were observed between the serum albumin level at 0600 h and 2200–0800 h AUC for glycemic variability or between the serum albumin level at 0600 h and 2200–0800 h UGE (r = − 0.213/0.401 and − 0.362/0.066, respectively). Furthermore, there were no significant correlations between the serum albumin level at 2100 h and 0800–2200 ho AUC for glycemic variability or between the serum albumin level at 2100 h and 0800–2200 h UGE (data not shown) (r = 0.051/0.221 and − 0.168/0.299, respectively).
Relationship between the area under the curve (AUC) for glycemic variability and urinary glucose excretion (UGE). Relationship between the 0800–2200 h AUC for glycemic variability and 0800–2200 h UGE or between 2200–0800 h AUC for glycemic variability and 2200–0800 h UGE shown in the upper and lower rows, respectively. For both tofogliflozin and ipragliflozin treatments, there was a significant negative correlation between 0800–2200 h AUC for glycemic variability and 0800–2200 h UGE (r = 0.441 and 0.480, respectively). In contrast, there was no significant correlation between 2200–0800 h AUC for glycemic variability and 2200–0800 h UGE (r = 0.282 and 0.212, respectively). These associations were analyzed using Pearson’s product moment correlation coefficient
Discussion
The present study showed that the efficacy and safety of tofogliflozin were significantly higher than those of ipragliflozin in patients with T2DM treated with basal insulin along with oral agents via insulin glargine U300. The objective was to achieve preprandial glucose levels of 80 to < 110 mg/dl. We made the aforementioned comparisons using the FGM data. The study was conducted in a single center using a moderate sample size.
Suppressing hypoglycemia reduces mortality [5], whereas reducing glycemic variability lowers the incidence of cardiovascular events and mortality [6]. Thus, it is preferable to select SGLT2 inhibitors that reduce hypoglycemia and glycemic variability. The significant differences between tofogliflozin and ipragliflozin in terms of their efficacy and safety indices may be explained by the differences in their half-life, protein-binding rate, and unaltered SGLT2 inhibitor excretion.
The SGLT2 inhibitors promote urinary glucose excretion and lower blood glucose by selectively inhibiting SGLT2, which reabsorbs glucose from the renal proximal tubules. SGLT2 expression may be comparatively elevated in patients with T2DM. Hence, they present with augmented renal tubular glucose reabsorption capacity and elevated glucose excretion threshold and urinary glucose level. In these patients, the blood glucose level exceeds the glucose excretion threshold after each meal [36]. SGLT2 inhibitors lower postprandial glucose levels and reduce glycemic variability.
Here, tofogliflozin lowered the glucose levels 2 h after each meal to a significantly greater extent than ipragliflozin. Postprandial glucose levels mainly affect glycemic variability [37]. The relative differences in the postprandial glucose level caused by the two SGLT2 inhibitors indicated that tofogliflozin significantly lowers 24-SD of glycemic variability and MAGE than ipragliflozin. The protein-binding rate of tofogliflozin was 83%, whereas that of ipragliflozin was between 94.6% and 96.5%. The excretion rate of unmetabolized tofogliflozin was 18.1%, whereas that of ipragliflozin was only 1%. The half-life of tofogliflozin is 5.4 h, whereas that of ipragliflozin is 15 h [38,39,40]. In the blood, these drugs are bound to plasma proteins such as albumin. SGLT2 inhibitors affect the renal proximal tubules. SGLT2 is present even after renal glomerular filtration. The relatively low protein-binding rate of tofogliflozin indicates that comparatively more tofogliflozin is filtered through the glomerulus and reaches the renal tubules. Both SGLT2 inhibitors are metabolized mainly in the liver but the unmetabolized SGLT2 inhibitors are filtered in the renal glomeruli and act on the renal tubules. Therefore, as tofogliflozin has a low protein-binding rate and a short half-life, its diurnal filtration through the renal glomerulus was higher than that of ipragliflozin. Comparatively more unchanged tofogliflozin was excreted and it suppressed postprandial glucose levels. SGLT2 inhibitors suppress glycemic variability by reducing the postprandial glucose level. However, the 0800–2200 h AUC for glycemic variability and 0800–2200 h UGE were significantly negatively correlated for both tofogliflozin and ipragliflozin. Contrarily, there was no significant correlation between the 2200–0800 h AUC for glycemic variability and 2200–0800 h UGE. This result can be attributed to the direct pharmacologic action of SGLT2 inhibitors. We found no significant correlations between the serum albumin level and AUC for glycemic variability or UGE. Numerous drugs bind to plasma proteins such as albumin. When they are released from the protein, they exert their effects. Hence, we predicted that the relative differences in protein binding between tofogliflozin and ipragliflozin influence their hypoglycemic effects. However, the amount of albumin is considerably higher than that of the drug itself. The concentration of drug released from the protein increased at the serum albumin level of ≤ 3.5 g/dl. The increase was especially significant at ≤ 3.0 g/dl [41]. Here, the serum albumin levels were not low. Thus, there was no apparent correlation between the hypoglycemic effects of the drug and serum albumin level. Nevertheless, SGLT2 inhibitors might be potentiated under hypoalbuminemia caused by nephrotic syndrome or cirrhosis.
There was no significant difference between tofogliflozin and ipragliflozin in terms of 24-h UGE. Contrarily, the 0800–2200 h UGE was significantly higher in response to tofogliflozin than to ipragliflozin administration. Conversely, the 2200–0800 h UGE after ipragliflozin administration was significantly higher than that after tofogliflozin administration. This discrepancy can be attributed to the difference between these two drugs in terms of half-life. The half-life of tofogliflozin is 5.4 h, whereas that of ipragliflozin is 15–16 h [38,39,40]. Therefore, when tofogliflozin is administered in the morning, it induces a stronger diurnal UGE effect than ipragliflozin, and this response decreases at night. In contrast, as ipragliflozin has a long half-life, its influence on diurnal urinary glucose excretion is weaker than that of tofogliflozin, but the effect of the former continues until night [42]. The wide difference in the half-life between tofogliflozin and ipragliflozin caused a significant difference between the incidence of the safety assessment index TBR level 1 (especially nocturnal) and that of the safety assessment index TBR level 2. We used insulin glargine U300 to titrate preprandial glucose level at breakfast to between 80 and < 110 mg/dl. The preprandial glucose level did not significantly differ between the tofogliflozin and ipragliflozin treatments. However, TBR level 1 was significantly higher for ipragliflozin (8.7% ± 11.7%) than for tofogliflozin, and it manifested mainly as nocturnal (0000–0600 h) hypoglycemia (5.6% ± 7.7%). We previously reported that insulin glargine U300 is relatively less likely to induce nocturnal hypoglycemia [27]. There were significant differences between tofogliflozin and ipragliflozin in terms of nocturnal hypoglycemia induction risk because of the differences in their pharmacokinetics (half-life). Nocturnal hypoglycemia attenuates sympathetic nerve conduction, triggers bradyarrhythmia, and increases cardiovascular disease risk [43]. Therefore, tofogliflozin may be comparatively safer than ipragliflozin as the former is less likely to cause nocturnal hypoglycemia.
The target HbA1c level recommended to prevent diabetes-related complications varies with patients’ heath status, age, and social history [44]. As the HbA1c level reflects the average glucose level over 2–3 months, it is difficult to evaluate diurnal glycemic variability. Contrarily, FGM reveals glycemic variability in real time and indicates the frequency and severity of hypoglycemia and hyperglycemia under the current treatment. Thus, FGM may prove efficacious in diabetes therapy as it compensates for insufficient HbA1c level. Here, tofogliflozin and ipragliflozin achieved > 70% of the recommended target TIR range in patients with T2DM who were neither elderly nor at a high risk. However, in terms of TBR level 1 and 2 hypoglycemia, ipragliflozin did not achieve the target ranges of < 4% and < 1%, respectively. Ipragliflozin is more likely to cause nocturnal hypoglycemia than tofogliflozin [45]. For this reason, tofogliflozin administration may be preferable for patients at a high risk of hypoglycemia.
There were some limitations to this study. This was a crossover study, with a small sample size. Furthermore, the study was conducted in a single center. Thus, the sample size must be increased over 100 patients and the study must be conducted in more than 10 centers using a common protocol to verify whether the results obtained here are realistic. Moreover, the duration of the study was short. To evaluate long-term efficacy and safety, an extended crossover study for > 1 year should be conducted and FGM should be examined during each drug administration period. In this manner, it will be possible to establish whether the suppression of glycemic variability and hypoglycemia can effectively mitigate diabetes-related complications and attenuate cardiovascular events. Finally, as the aim of this study was to evaluate the efficacy and safety of SGLT2 inhibitors, the selection criteria were normal-to-moderate renal dysfunction for which SGLT2 inhibitors were effective. However, as the risk of cardiovascular disease is higher in patients with more advanced renal dysfunction, it remains unclear whether SGLT2 inhibitors can reduce the risk of cardiovascular diseases in such patients.
Conclusion
In conclusion, data collected by FGM in patients with T2DM treated with insulin glargine U300 demonstrated that tofogliflozin was more effective and safer than ipragliflozin in reducing diurnal glycemic variability and lowering the risk of nocturnal hypoglycemia.
References
American Diabetes Association. Standards of medical care in diabetes-2020 abridged for primary care providers. Clin Diabetes. 2020;38:10–38. https://doi.org/10.2337/cd20-as01.
Baena-Díez JM, Peñafiel J, Subirana I, et al. Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care. 2016;39:1987–95. https://doi.org/10.2337/dc16-0614.
Hanefeld M, Temelkova-Kurktschiev T. Control of post-prandial hyperglycemia—an essential part of good diabetes treatment and prevention of cardiovascular complications. Nutr Metab Cardiovasc Dis. 2002;12:98–107.
Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. New Engl J Med. 2010;363:1410–8. https://doi.org/10.1056/NEJMoa1003795.
Fisman EZ, Motro M, Tenenbaum A, et al. Is hypoglycaemia a marker for increased long-term mortality risk in patients with coronary artery disease? An 8-year follow-up. Eur J Cardiovasc Prev Rehabil. 2004;11:135–43. https://doi.org/10.1097/01.hjr.0000124326.85096.ec.
Cavalot F, Pagliarino A, Valle M, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34:2237–43. https://doi.org/10.2337/dc10-2414.
American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in Diabetes-2020. Diabetes Care. 2020;43:S98–110. https://doi.org/10.2337/dc20-S009.
Brunton S. GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int J Clin Pract. 2014;68:557–67. https://doi.org/10.1111/ijcp.12361.
Hollander PA, Kushner P. Type 2 diabetes comorbidities and treatment challenges: rationale for DPP-4 inhibitors. Postgrad Med. 2010;122:71–80. https://doi.org/10.3810/pgm.2010.05.2144.
Scheen AJ. SGLT2 inhibitors: benefit/risk balance. Curr Diabetes Rep. 2016;16:92. https://doi.org/10.1007/s11892-016-0789-4.
Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5:355–66. https://doi.org/10.1007/s11892-016-0789-4.
Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–74.
Zhao Y, Xu L, Tian D, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20:458–62. https://doi.org/10.7326/0003-4819-159-4-201308200-00007.
Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61:2098–107. https://doi.org/10.1007/s00125-018-4669-0.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New Engl J Med. 2015;373:2117–28. https://doi.org/10.1056/NEJMoa1504720.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New Engl J Med. 2017;377:644–57. https://doi.org/10.1056/NEJMoa1611925.
Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. New Engl J Med. 2013;369:1317–26. https://doi.org/10.1056/NEJMoa1307684.
White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. New Engl J Med. 2013;369:1327–35. https://doi.org/10.1056/NEJMoa1305889.
Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. New Engl J Med. 2015;373:232–42. https://doi.org/10.1056/NEJMoa1501352.
Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. https://doi.org/10.1111/j.1463-1326.2011.01517.x.
Keen P. Effect of binding to plasma proteins on the distribution, activity and elimination of drugs. In: Brodie BB, Gillette JR, Ackerman HS, editors. Concepts in biochemical pharmacology: part 1. Berlin: Springer; 1971. p. 213–33.
Gonzalez D, Schmidt S, Derendorf H. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin Microbiol Rev. 2013;26:274–88. https://doi.org/10.1128/CMR.00092-12.
Ikeda S, Takano Y, Cynshi O, et al. A novel and selective sodium-glucose cotransporter-2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:984–93. https://doi.org/10.1111/dom.12538.
Yamada K, Nakayama H, Yoshinobu S, et al. Effects of a sodium glucose co-transporter 2 selective inhibitor, ipragliflozin, on the diurnal profile of plasma glucose in patients with type 2 diabetes: a study using continuous glucose monitoring. J Diabetes Investig. 2015;6:699–707. https://doi.org/10.1111/jdi.12370.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–603. https://doi.org/10.2337/dci19-0028.
Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65. https://doi.org/10.1186/1475-2840-13-65.
Kawaguchi Y, Sawa J, Sakuma N, Kumeda Y. Efficacy and safety of insulin glargine 300 U/mL vs insulin degludec in patients with type 2 diabetes: a randomized, open-label, cross-over study using continuous glucose monitoring profiles. J Diabetes Investig. 2019;10:343–51. https://doi.org/10.1111/jdi.12884.
Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787–94. https://doi.org/10.1089/dia.2014.0378.
American Diabetes Association. Postprandial blood glucose. Diabetes Care. 2001;24:775–8. https://doi.org/10.2337/diacare.24.4.775.
Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–40. https://doi.org/10.2337/dc17-1600.
Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability: does it matter? Endocr Rev. 2010;31:171–82. https://doi.org/10.1210/er.2009-0021.
Nishimura R, Osonoi T, Kanada S, et al. Effects of luseogliflozin, a sodium–glucose co-transporter 2 inhibitor, on 24-h glucose variability assessed by continuous glucose monitoring in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, crossover study. Diabetes Obes Metab. 2015;17:800–4. https://doi.org/10.1111/dom.12481.
Samukawa Y, Omiya H, Watase H, Nozaki K, Sakai S, Nishimura R. Substantial effects of luseogliflozin revealed by analyzing responses to postprandial hyperglycemia: post hoc subanalyses of a randomized controlled study. Adv Ther. 2016;33:1215–30. https://doi.org/10.1007/s12325-016-0350-5.
Jones B, Kenward MG. Design and analysis of cross-over trials. 3rd ed. Abingdon: Routledge; 1989.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244.
DeFronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013;36:3169–76. https://doi.org/10.2337/dc13-0387.
Levetan C, Want LL, Weyer C, et al. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care. 2003;26:1–8. https://doi.org/10.2337/diacare.26.1.1.
Poole RM, Dungo RT. Ipragliflozin: first global approval. Drugs. 2014;74:611–7. https://doi.org/10.1007/s40265-014-0204-x.
Kasahara-Ito N, Fukase H, Ogama Y, et al. Pharmacokinetics and pharmacodynamics of tofogliflozin (a selective SGLT2 inhibitor) in healthy male subjects. Drug Res. 2017;67:349–57. https://doi.org/10.1055/s-0043-104779.
Gu N, Park S-I, Chung H, Jin X, Lee SH, Kim TE. Possibility of pharmacokinetic drug interaction between a DPP-4 inhibitor and a SGLT2 inhibitor. Transl Clin Pharmacol. 2020;28:17–33. https://doi.org/10.12793/tcp.2020.28.e4.
Gugler R, Azarnoff DL, Shoeman DW. Diphenylhydantoin: correlation between protein binding and albumin concentration. Wien Klin Wochenschr. 1975;53:445–6. https://doi.org/10.1007/BF01493371.
Takeishi S, Tsuboi H, Takekoshi S. Comparison of tofogliflozin 20 mg and ipragliflozin 50 mg used together with insulin glargine 300 U/mL using continuous glucose monitoring (CGM): a randomized crossover study. Endocr J. 2017;64:995–1005. https://doi.org/10.1507/endocrj.EJ17-0206.
Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63:1738–47. https://doi.org/10.2337/db13-0468.
American Diabetes Association. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S66–76. https://doi.org/10.2337/dc20-S006.
Kurozumi A, Okada Y, Shimokawa M, et al. Efficacy and safety of tofogliflozin on 24-h glucose profile based on continuous glucose monitoring: crossover study of sodium-glucose cotransporter 2 inhibitor. Diabetes Technol Ther. 2019;21:385–92. https://doi.org/10.1089/dia.2019.0099.
Acknowledgements
We thank all participants of the present study, the Minami Osaka Hospital staff for their assistance, and Editage (https://www.editage.com) for English language editing.
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s Rapid Service Fee was funded by the authors.
Authorship
Conception and design of study: Y. Kawaguchi; data acquisition: Y. Kawaguchi, J. Sawa; analysis and interpretation of data: Y. Kawaguchi, J. Sawa; drafting of manuscript: Y. Kawaguchi. All authors have approved the final article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and take responsibility for the integrity of the work as a whole. All authors provided their approval for this version of the manuscript to be published.
Disclosures
Y. Kawaguchi received lecture honoraria or speaker fees from Sanofi K.K., Novo Nordisk Pharma, Takeda Pharmaceutical Co., Ono Pharmaceutical Co., Boehringer Ingelheim, Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc., and Kowa Company Ltd. The remaining authors Jun Sawa and Yasuro Kumeda have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by the Minami Osaka Hospital Ethics Committee (no. 2018-16) and registered in the University Hospital Medical Information Network Clinical Trial Registry (no. UMIN000037158). The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All participants were briefed on the study outline before their participation, and they provided written informed consent.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kawaguchi, Y., Sawa, J. & Kumeda, Y. Efficacy and Safety of Tofogliflozin and Ipragliflozin for Patients with Type-2 Diabetes: A Randomized Crossover Study by Flash Glucose Monitoring. Diabetes Ther 11, 2945–2958 (2020). https://doi.org/10.1007/s13300-020-00940-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00940-9