Abstract
Introduction
There are a number of options for the symptomatic treatment of peripheral neuropathy, but the overall treatment outcomes remain unsatisfactory.
Methods
A total of 60 patients with refractory diabetic neuropathy were randomly assigned to two groups. Patients in Group A were treated with computed tomography (CT)-guided sympathetic neurolysis with alcohol, and patients in Group B were treated with a combined therapy of CT-guided catheterization to achieve continuous lumbar block for 4 weeks followed by neurolysis with alcohol administered via the catheter. The outcomes of these two treatment strategies were then analyzed in terms of pain relief, blood flow in the lower limb microcirculation, plasma levels of inflammatory mediators, and complications.
Results
The visual analog scale (VAS) pain scores of all patients after treatment decreased significantly at the different evaluation time points compared with pre-treatment values, with the intergroup analysis revealing that the VAS scores were lower in Group B patients than in Group A patients at all post-treatment time points. Skin temperature, capillary filling time, and blood oxygen saturation level were significantly improved in all patients at the 1- and 7-day post-treatment assessment compared to pre-treatment values, but patients in Group B showed a greater improvement. The plasma levels of inflammatory mediators were lower in all patients at the 7-day post-treatment assessment compared to pre-treatment values, with those of patients in Group B being statistically significantly lower than those of patients in Group A.
Conclusion
Combined treatment with continuous lumbar sympathetic block followed by neurolysis with alcohol provided more benefit in all assessed outcomes than sympathetic alcohol neurolysis alone. The results show that the procedures were associated with satisfactory safety outcomes and sustained analgesic effects, thereby providing clinical evidence supporting the use of this novel treatment for patients with painful diabetic neuropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are a number of options for symptomatic treatment of peripheral neuropathy, but the overall outcome is still not satisfactory. |
We have evaluated the outcomes of sympathetic alcohol neurolysis in a cohort of patients with painful diabetic neuropathy in terms of pain relief, blood flow in the lower limb microcirculation, plasma levels of inflammatory mediators, and complications. |
The results show that the treatments have acceptable safety outcomes and provide satisfactory pain relief to patients. |
These therapeutic approaches may provide additional management options for patients in the future. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12854498.
Introduction
Peripheral neuropathy is one of the most common complications of patients with type 1 or type 2 diabetes [1], with an estimated prevalence in this patient population of approximately 30% [2]. It is also a leading cause of morbidity and is associated with increased mortality [2]. Symptoms of peripheral neuropathy include loss of sensation, neuropathic pain, and paresthesia [1, 3]. The loss of sensation in patients with diabetic peripheral neuropathy (DPN) may lead to ulcers, which is a major cause of amputation [4]. Many of the symptoms of the neuropathy can last for years and severely impact the affected individual’s quality of life.
The management and treatment of DPN remain challenging to both patients and clinicians [5, 6]. At the present time there is no satisfactory cure for the disease, and treatment strategy focuses on slowing the progress of the neuropathy, relieving pain, and managing complications. Patients are recommended to have a healthy eating plan and adopt a healthy life style to help keep the blood sugar level within a target range. Several medications are used to relieve pain, with first-line treatment options including tricyclic antidepressants (e.g., amitriptyline, nortriptyline) [7]. Serotonin–norepinephrine reuptake inhibitors (e.g., venlafaxine, duloxetine, or opiates and opiate analogs) may also be used if other first-line treatments are not successful [7]. While a number of treatments are available, the overall outcomes of the current management are still not satisfactory [8], and new treatments of diabetic neuropathy are continuously being investigated.
More recently, non-pharmacological approaches, such as spinal cord stimulation, have been proposed to treat painful DPN. There have been reports that spinal cord stimulation can improve diabetic limb circulation blood flow and relieve neuropathic and ischemic pain [9, 10]. Improvement of the peripheral blood flow may be involved in the mechanism of the analgesic effect of spinal cord stimulation. In China, spinal cord stimulation for treating neuropathic pain is not in wide use due to the high cost of the treatment; as alternative, another non-pharmacological approach, lumbar sympathetic blockade, has received much interest [11]. Lumbar sympathetic blockade has the advantage of being a minimally invasive technique, with patients usually having a quick recovery time. In addition, the treatment is affordable to the majority of patients. Although the technique has been available for a long time, its safety and outcomes in patients with painful diabetic peripheral neuropathy have not been intensively studied. One case report suggested that sympathetic blocks can be an effective management of painful diabetic neuropathy [11].
In the study reported here, we evaluated the outcomes of two treatment variations with sympathetic neurolysis with alcohol in a cohort of patients with painful diabetic neuropathy with the overall aim to determine whether the treatments have acceptable safety outcomes and provide satisfactory pain relief to patients. These therapeutic approaches may provide additional management options for patients in the future.
Methods
A total of 60 adult patients with refractory diabetic neuropathy were enrolled in this study. DPN was diagnosed in accordance with the guidelines published by the American Diabetes Association [12]. Briefly, the diagnosis was made based on the clinical history of the patient and/or the presence of overt neuropathic symptoms, and/or neurologic examinations. The latter included the 10 g Semmes–Weinstein monofilament test; the 128-Hz tuning fork examination; temperature and pain (pinprick) sensations; and ankle/heel reflexes. If two or more of these tests were determined to be abnormal, the patient was considered to have diabetic neuropathy. Other causes of neuropathies (such as lumbar disc herniation, central post-stroke pain, entrapments, fasciitis, and Guillain–Barré syndrome; alcohol abuse) were excluded. Patients with signs of significant cardiovascular diseases, morbid obesity, and/or vital organ dysfunction, those recently receiving medications known to affect blood pressure and heart rate, or those with a predicted difficulty in airway management were excluded. The study was approved by the ethics committee of Beijing Anzhen Hospital (No. 2012002X), and written informed consent was obtained from all of the participating patients. All procedures performed in the studies were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Patients were randomly assigned into two groups. Group A consisted of patients receiving a single lumbar sympathetic ganglion block (n = 30), and Group B comprised patients who received combination therapy consisting of a continuous lumbar sympathetic ganglion block with anti-inflammatory medication and neurolytic therapy (n = 30). In Group A, patients were given oxycodone hydrochloride tablets (10 mg, every 12 h) to control the pain. The patient was placed in the prone position on the flat bed of the computed tomography (CT) machine and the vital signs continuously monitored. A lumbar sympathetic nerve block was performed using a standard posterolateral approach by inserting an 18-gauge Tuohy needle into the corresponding sympathetic trunk under CT guidance. This was followed by the injection of 20 mL of 1% lidocaine and iohexol mixture to confirm the successful puncture. Changes in muscle strength in the lower limbs were monitored before and after the operation for at least 30 min; if the muscle strength had not been decreased by the nerve block test, 5 mL of 95% anhydrous ethanol was then slowly injected. In Group B, patients were also given oxycodone hydrochloride tablets to control the pain. After insertion of the needle and confirmation of its position with the CT contrast agent, a catheter was inserted into the corresponding sympathetic trunk and then connected to a patient-controlled analgesia (PCA) pump. A mixture of dexamethasone and lidocaine (0.4% lidocaine + 0.008% dexamethasone) was pumped continuously. The basal dose for PCA was 5 mL/h, while the bolus dose was 1 mL with a lockout time of 15 min. The duration of the continuous block was 4 weeks, following which 5 mL of 95% anhydrous ethanol was injected as described above for Group A.
Pain Evaluation, Laboratory Results, and Complications
A visual analog scale (VAS) was used to assess the intensity of pain (range: 0, indicating no pain, to 10, indicating worst pain). The assessments were performed before treatment and at 1 and 7 days and 6 months post-treatment. The blood supply to the limbs was evaluated by measuring skin temperature, capillary refill time, and percutaneous oxygen saturation. All of the measurements were performed before treatment and at 1 and 7 days post-treatment.
Plasma levels of pain and inflammatory mediators, including norepinephrine, serotonin, substance P, and β-endorphin, were evaluated on days 1 and 7 post-treatment. Blood samples for these assessments were collected from patients who had fasted for 12 h. The consumption of food or drinks with high caffeine or glucose contents (e.g., chocolate, coffee, tea, apricots, bananas, etc.) was prohibited.
Any complications, including urination, intestinal motility disorders, or others, during the duration of hospital stay or during follow-up period were recorded.
Statistical Analysis
Statistical analyses were performed using SPSS statistical software (IBM Corp., Armonk, NY, USA). The quantitative data are expressed as the mean ± standard deviation . The paired sample t test was used to compare differences between two groups, one-way analysis of variance was used to analyze values in the clinical course within a group, and the Chi-squared tests were used to compare differences in frequencies. A p value < 0.05 was considered to be statistically significant.
Results
Patient Characteristics
A total of 60 patients (34 men, 26 women) participated in the current study. The mean age of the patient cohort was 64 (range 37–88) years. Patients were randomly assigned to Group A (n = 30) or Group B (n = 30). The baseline VAS for the intensity of pain at baseline reported by these patients ranged from 7 to 10. There were no significant differences between these two groups of patients in terms of demographic characteristics and baseline VAS.
Pain Relief Outcomes
The assessment of pain was performed before treatment and at several time points post-treatment, namely, on days 1 and 7 and months 1 and 6 post-surgery. The mean post-treatment VAS fell significantly in both treatment groups (Table 1). For patients in Group A, the score fell from 8.5 pre-treatment to post-treatment values of 2.9 on day 1, 3.1 on day 7, 4.2 at 1 month, and 4.9 at 6 months; for patients in Group B, the score fell from 9.1 pre-treatment to post-treatment values of 2.2 on day 1, 2.6 on day 7, 2.7 at 1 month, and 4.1 at 6 months. A comparison of Group A and Group B VAS scores revealed that the decrease in VAS score was greater in Group B patients at all post-treatment assessment time points (days 1 and 7; months 1 and 6) (Fig. 1).
Blood Supply to the Limbs and Laboratory Results
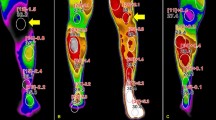
Skin temperature, capillary refill time, and percutaneous oxygen saturation were measured before treatment and at 1 and 7 days post-treatment. All patients showed significant improvement in these parameters immediately after treatment (Table 2). Comparison of Group A and Group B revealed that the improvement was greater in Group B. The skin temperature of patients in Group A increased by 6.3 and 2.7% at 1 and 7 days post-treatment, respectively, and that of patients in Group B increased by 6.9 and 4%, respectively.
The plasma levels of pain and inflammatory mediators were measured at pre-treatment and at 7 days post-treatment. The levels of all pain and inflammatory mediators, including β-endorphin, norepinephrine, serotonin, and substance P, had fallen significantly at 7 days post-treatment compared to baseline (pre-treatment) (p < 0.05) (Table 3). Comparison of Group A and Group B revealed that the plasma levels of all mediators were significantly lower in patients in Group B (p < 0.05).
Post-Treatment Safety Follow-Up
Safety follow-ups were performed on days 1 and 7 and 1 month post-treatment to observe any complications. No patient showed any indication of urination, ejaculation, and bowel dysfunction. In Group A, three patients reported lower back pain for 2 days. In addition, one patient reported lower limb muscle weakness after the ethanol injection; this patient was immediately injected with saline and the symptoms resolved. Also, one patient presented numbness of the anterior thigh, but the symptoms disappeared 1 week after the treatment. In Group B, only two patients minor complications, consisting of mild skin allergies in the lower limbs during the stay for microcatheter delivery. Overall, the complication rate in Group A and Group B was 0.17 and 0.1%, respectively.
Discussion
Diabetic neuropathy pain management remains a challenge, and the overall outcomes of current pain management in diabetic neuropathy need to be improved [6]. Lumbar sympathetic block has been used to diagnose [13] and/or treat various types of pain, including visceral pain and complex regional pain syndrome I [14,15,16,17]. However, only a limited number of studies have explored this strategy for treating painful diabetic neuropathy. In our study we observed that lumbar sympathetic blockade significantly reduced neuropathic pain in all patients (both Group A and Group B) and that the pain relief was sustained at 6 months post-treatment, suggesting that the treatment has long-lasting analgesic effects.
It is recognized that the sympathetic nervous system may play an important role in painful diabetic neuropathy. In one study, the level of circulating norepinephrine was higher in patients with painful versus painless diabetic neuropathy, suggesting that a relatively higher number of functioning sympathetic fibers may contribute to pain [18]. Substance P is considered to be a primary neurotransmitter of painful stimuli to the central nervous system. Studies have shown significant pain reduction in patients with diabetic neuropathy following treatment with capsaicin cream, a treatment which depletes substance P [19]. In our study, we also observed a dramatic decrease in the plasma levels of norepinephrine and substance P in patients after treatment (Table 3). Overall, our results suggest that the use of sympathetic nerve blocks may reduce the pain by reducing sympathetic outflow.
Patients with painful diabetic neuropathy exhibit vasoconstriction, suggesting that inappropriate local blood flow regulation may play a role in the pathogenesis of pain [20]. In the present study, we observed a significant improvement of microvascular circulation, as indicated by the increase in skin temperature, reduction of capillary refill time, and increase in percutaneous oxygen saturation (Table 2). This improvement of microvascular circulation may contribute to the pain relief in patients.
Our treatment protocol in Group B patients also included the use of the anti-inflammatory substances dexamethasone and lidocaine. The addition of these anti-inflammatory medications to the treatment regimen appeared to improve the analgesic effects of the lumbar sympathetic blockade. It has consistently been shown that the administration of dexamethasone can prolong analgesia after peripheral nerve block [21]. One case report showed that the use of a mixture of lidocaine and triamcinolone in a lumbar sympathetic block was able to significantly reduce the pain scores of the patient with painful diabetic neuropathy [11]. It has also been suggested that damaged nerve fibers often have dysregulated expression of sodium channels that are particularly sensitive to local anesthetics, such as lidocaine [22]. The sympathetic blocks with steroids and a local anesthetic may provide pain relief through actions similar to the pharmacological effect on nociceptive fibers.
Lumbar sympathetic blockade generally is a low-risk procedure. The most common complication associated with lumbar sympatholysis is neuralgia of the genitofemoral nerve, particularly in surgery involving the lateral approach. Most of the complications are transient and can be resolved with nonprescription analgesics. Bleeding may also occur in patients with a clotting deficiency, which should be evaluated before treatment. Otherwise, any bleeding from needle puncture should be self-limiting. Patients should also be informed that hypotension may occur after sympatholysis, and men may experience a failure of ejaculation, although such complications were not observed in the current study. Overall, we observed that the procedures had a satisfactory safety level and a low rate of complications.
The study has a number of limitations. First, additional tools (e.g., nerve conduction study, electromyogram) should be used to diagnose and assess the severity and duration of DPN. Second, the population size was relatively small. Third, the follow-up period was short (6 months), and thus the long-term outcome (> 9 months) has not been studied. Last, other methods of improving microcirculation and the use of analgesic pumps alone should have been used as a control group to more rigorously compare the effects of lumbar sympathetic block.
While recognizing the limitations of the current study, the positive outcomes of the treatment warrants further investigation. Given the inefficacy of current treatments, our study provides insights for novel treatment options for patients with painful diabetic neuropathy.
Conclusions
In conclusion, the procedure of CT-guided continuous lumbar sympathetic block with alcohol neurolysis provided significant pain relief for patients with painful diabetic neuropathy. The analgesic effects of the continuous blocks were reproducible and long-lasting. The procedures had satisfactory safety, and patients recovered quickly, providing clinical evidence that the pain in diabetic neuropathy may be sympathetically mediated to a significant extent. While recognizing the limitations to our study (described above), we suggest that the positive outcomes of the treatment described here warrants further investigation and that the study provides insights for novel treatment options for patients with painful diabetic neuropathy.
References
Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–22.
Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–34.
Poncelet AN. Diabetic polyneuropathy risk factors, patterns of presentation, diagnosis, and treatment. Geriatrics. 2003;58(6):16–8, 24–5, 30.
Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes Metab Res Rev. 2004;20(Suppl 1):S13–8.
Pluijms W, Huygen F, Cheng J, et al. Evidence-based interventional pain medicine according to clinical diagnoses. 18. Painful diabetic polyneuropathy. Pain Pract. 2011;11(2):191–8.
Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9(6):660–74.
Lindsay TJ, Rodgers BC, Savath V, Hettinger K. Treating diabetic peripheral neuropathic pain. Am Fam Physician. 2010;82(2):151–8.
Javed S, Petropoulos IN, Alam U, Malik RA. Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis. 2015;6(1):15–28.
McGreevy K, Williams KA. Contemporary insights into painful diabetic neuropathy and treatment with spinal cord stimulation. Curr Pain Headache Rep. 2012;16(1):43–9.
Pluijms WA, Slangen R, Joosten EA, et al. Electrical spinal cord stimulation in painful diabetic polyneuropathy, a systematic review on treatment efficacy and safety. Eur J Pain. 2011;15(8):783–8.
Cheng J, Daftari A, Zhou L. Sympathetic blocks provided sustained pain relief in a patient with refractory painful diabetic neuropathy. Case Rep Anesthesiol. 2012;2012:285328.
American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl 1):S12–54.
An JW, Koh JC, Sun JM, et al. Clinical identification of the vertebral level at which the lumbar sympathetic ganglia aggregate. Korean J Pain. 2016;29(2):103–9.
Tang YZ, Shanno ML, Lai GH, Li XY, Li N, Ni JX. Anterior herniation of lumbar disc induces persistent visceral pain: discogenic visceral pain: discogenic visceral pain. Chin Med J (Engl). 2013;126(24):4691–5.
Hong JH, Oh MJ. Comparison of multilevel with single level injection during lumbar sympathetic ganglion block: efficacy of sympatholysis and incidence of psoas muscle injection. Korean J Pain. 2010;23(2):131–6.
Tang YZ, Ni JX, An JX. Complex regional pain syndrome type I following discTRODE radiofrequency treated with continuous lumbar sympathetic trunk block using patient-controlled analgesia. Pain Med. 2013;14(2):309–10.
Ruben JE. Continuous lumbar sympathetic block for the treatment of acute arterial occlusion and other vascular diseases of the lower extremity. Ann Surg. 1950;131(2):194–205.
Tsigos C, Reed P, Weinkove C, White A, Young RJ. Plasma norepinephrine in sensory diabetic polyneuropathy. Diabetes Care. 1993;16(5):722–7.
Ziegler D. Painful diabetic neuropathy: advantage of novel drugs over old drugs? Diabetes Care. 2009;32(Suppl 2):S414–9.
Quattrini C, Harris ND, Malik RA, Tesfaye S. Impaired skin microvascular reactivity in painful diabetic neuropathy. Diabetes Care. 2007;30(3):655–9.
Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS ONE. 2015;10(9):e0137312.
Craner MJ, Klein JP, Renganathan M, Black JA, Waxman SG. Changes of sodium channel expression in experimental painful diabetic neuropathy. Ann Neurol. 2002;52(6):786–92.
Acknowledgements
We thank the participants of the study.
Funding
This work was supported by Beijing Science and Technology Project (no. Z121107001012159), Beijing Health System High-level Health Technical Talents Cultivation Fund (no. 2013-2-004), Capital Medical Development Research Fund (no. 2009-3145), the National Natural Science Foundation of China (No. 30972851), and the National Natural Science Foundation of China (no. 61875141). The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Haiyan Sun and Jun Ma contributed to the conception and design of the study; Mingwei He, Jinlei Pang, Xiangfei Guo and Yansong Huo performed the experiments, collected and analyzed data; Haiyan Sun and Jun Ma wrote the manuscript; All authors reviewed and approved the final version of the manuscript.
Disclosures
Haiyan Sun, Mingwei He, Jinlei Pang, Xiangfei Guo, Yansong Huo and Jun Ma have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the ethics committee of Beijing Anzhen Hospital (No. 2012002X), and written informed consent was obtained from all of the participating patients. All procedures performed in studies were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12854498.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sun, H., He, M., Pang, J. et al. Continuous Lumbar Sympathetic Blockade Enhances the Effect of Lumbar Sympatholysis on Refractory Diabetic Neuropathy: A Randomized Controlled Trial. Diabetes Ther 11, 2647–2655 (2020). https://doi.org/10.1007/s13300-020-00918-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00918-7