Abstract
Introduction
To evaluate the efficacy and safety of dipeptidyl peptidase 4 inhibitors (DPP4i) used in combination with insulin in patients with type 2 diabetes mellitus (T2DM).
Methods
We searched the MEDLINE, Embase, and Cochrane library databases for randomized controlled trials (RCTs) published through June 2018. Studies with at least a 12-week treatment period were included to compare the addition of DPP4i to insulin with insulin control therapy. Meanwhile, groups on a stable insulin dosage (insulin-stable subgroup) or titrating insulin dosage (insulin-flexible subgroup) were analyzed separately.
Results
Twenty-one RCTs with 3697 patients randomized to a DPP4i/insulin treatment arm and 3538 to an insulin control arm were included. DPP4i, when added to insulin therapy, led to a significantly greater reduction in HbA1c (− 0.57%, 95% CI − 0.66, − 0.48) and provided significantly greater odds of achieving the HbA1c target < 7% (OR 3.45; 95% CI 2.58, 4.63). These effects were achieved in the context of a decrease in the daily insulin requirement, without increases in hypoglycemia risk and body weight, compared with the control treatment. Subgroup analysis showed control-adjusted reductions in HbA1c from baseline in the insulin-stable subgroup (− 0.64%; 95% CI − 0.74, − 0.53) and the insulin-flexible subgroup (− 0.43%; 95% CI − 0.56, − 0.30). Other results occurred similarly in both subgroups.
Conclusions
The addition of DPP4i to insulin is associated with a statistically significant reduction in glycemic control as measured by HbA1c, fasting plasma glucose, and 2-h postprandial glucose, without increasing the risk of hypoglycemia and weight gain. These conclusions were also observed in both stable-dose and flexible-dose insulin subgroups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Effect of DPP4i used in combination with insulin treatment in patients with T2DM remains unclear in real-world clinical practice. |
In this meta-analysis, 21 RCTs with 3697 patients randomized to a DPP4i/insulin treatment arm and 3538 to an insulin control arm were included. |
What was learned from the study? |
The addition of DPP4i to insulin is associated with a statistically significant reduction in glycemic control as measured by HbA1c, FPG, and PPG-2h, without increasing the risk of hypoglycemia and weight gain. |
These data allowed us to truly evaluate the efficacy and safety of DPP4i as add-on therapy to insulin in real-world clinical practice. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12788354.
Introduction
Type 2 diabetes mellitus (T2DM) is a serious health problem that affects people around the world. The latest estimates showed a global diabetes prevalence of 9.3% (463 million) of the world population in 2019, and the prevalence is expected to further increase to 10.2% (578 million) by the year 2030 [1]. The diabetes prevalence has been rising more rapidly in middle- and low-income countries. However, in some countries like China only a small proportion of patients with T2DM reach the target level of hemoglobin A1c (HbA1c) [2]. Poor glycemic control can lead to a variety of serious complications of T2DM, including cardiovascular disease and diabetic nephropathy, which are major causes of mortality [3]. Therefore, achieving the targeted glycemic control is a key element of diabetes management.
The T2DM treatment guidelines suggest early initiation of insulin and treatment intensification for patients who are not achieving glycemic goals [3]. On the other hand, with the progression of disease, insulin monotherapy sometimes fails to provide good glycemic results, or up-titration of insulin is required to achieve or maintain adequate glycemic control. Some concerns about insulin initiation have therefore been raised, such as the risk of hypoglycemia, weight gain, and barriers to insulin self-titration [4, 5]. Thus, the use of the combination of insulin treatment with oral antidiabetic drugs (OADs) is recommended to minimize side effects and obtain optimal glycemic control [6].
Dipeptide peptidase 4 enzyme inhibitors (DPP4i) are a class of OADs that prevent the degradation of gastrointestinal incretins glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), resulting in glycemic improvement [6]. Both randomized clinical trials (RCTs) and pooled studies have demonstrated that DPP4i offer an ideal therapeutic option, as they improve glycemic control with a low risk of hypoglycemia and neutral effect on body weight [7, 8]. DPP4i have been listed as a strategic option to minimize hypoglycemia for individuals with HbA1c above target [3]. A number of RCTs have evaluated the efficacy and safety of DPP4i as add-on therapy to insulin in patients with T2DM [9,10,11]. However, the results are inconsistent, which may be due to variations in study design, patient populations, and insulin regimens.
Four previous meta-analyses evaluated the efficacy and safety of the addition of a DPP4i to insulin therapy in patients with T2DM [12,13,14,15]. However, these studies (1) mixed active controls and placebo as comparators, which made the interpretation of the pooled results difficult; (2) only included patients who were on a stable insulin dose, which was not applicable to clinical practice, because combined therapy is often indicated in patients who do not achieve optimal glycemic control by insulin therapy and insulin dose titration is needed in these patients; or (3) only included patients on insulin monotherapy or insulin plus metformin, which may not be representative of the general population. Therefore, an updated systematic review and meta-analysis is needed to evaluate the efficacy and safety of DPP4i used in combination with insulin in patients with T2DM compared with insulin control treatment.
This meta-analysis evaluated the efficacy and safety of DPP4i used in combination with insulin in patients with T2DM and separately analyzed subgroups on a stable insulin dosage versus a titrating insulin dosage.
Methods
Data Sources and Search Strategies
Studies were identified by a literature search of MEDLINE (PubMed), Embase, and the Cochrane library through June 2018. The search terms were (DPP-4 OR DPP-4 OR “dipeptidyl peptidase-4 inhibitors” OR sitagliptin OR vildagliptin OR linagliptin OR saxagliptin OR alogliptin OR dutogliptin OR gemigliptin OR anagliptin OR gosogliptin OR teneligliptin OR trelagliptin OR omarigliptin) AND (“insulin” OR “basal insulin” OR “premix insulin” OR “CSII” OR “short-acting” OR “basal–bolus” OR “basal bolus” OR glargine OR detemir OR degludec OR NPH OR lispro OR aspart OR rapid insulin OR insulin analogue) AND (T2DM OR “type 2 diabetes mellitus” OR “type 2 diabetes”) AND (random OR randomly OR randomized). The searched studies were limited to clinical trials in human.
Eligibility and Exclusion Criteria for Study Selection
The eligibility criteria were (1) RCTs in adult patients with T2DM; (2) compared the addition of DPP4i to insulin therapy (either with regimens of basal insulin, basal and premeal bolus of insulin, or premixed insulin) with insulin controls, with or without background therapy with other OADs; (3) study duration at least 12 weeks; and (4) reported at least one clinical outcome of interest. Non-RCTs, studies in patients with type 1 diabetes, and duplicated results were excluded.
Data Extraction
Data were extracted by two independent investigators. The following data from each study were recorded: publication data (title, first author, year of publication), study design, baseline characteristics of the study population, drug regimen, treatment duration, efficacy outcomes [HbA1c, fasting plasma glucose (FPG), 2-hour postprandial glucose (PPG-2h), total daily insulin dose; the number of participants achieving the target HbA1c < 7%)] and safety outcomes (body weight and incidence of hypoglycemia). Disagreements were resolved by consensus with a third investigator.
Assessment of Quality of Included Studies
Two independent investigators assessed the quality of the included studies by using the Cochrane risk of bias tool in terms of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases [16]. Plot analysis and Egger’s test were used for assessment of publication bias.
Statistical Analysis
For the continuous variables, including the change in HbA1c, FPG, PPG-2h, body weight, and daily dosage of insulin from baseline, effect sizes were estimated using meta-analysis as weighted mean differences (WMDs) with 95% confidence intervals (95% CIs). For categorical outcomes such as the number of participants achieving the HbA1c goal and those having hypoglycemia, pooled odds ratios (ORs) were calculated by meta-analysis. For all meta-analyses, a random effects model with inverse-variance weights and DerSimonian-Laird measure for estimating heterogeneity (the between-study variance) were used. The I2 statistic was used to calculate the extent of heterogeneity across the selected studies. The meta-analysis was conducted using the R software. A p value less than 0.05 was considered statistically significant.
Compliance with Ethics Guidelines
Ethical approval was unnecessary in this study because it was a systematic review and meta-analysis of existing published articles and therefore does not contain any studies with human participants or animals performed by any of the authors.
Results
Description of Enrolled Studies
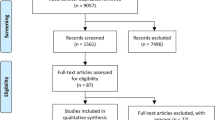
A total of 2630 published articles were retrieved, among which 21 RCTs with 3697 patients randomized to a DPP4i/insulin treatment arm and 3538 patients randomized to an insulin control arm were finally included for the meta-analysis [9,10,11, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The study selection process is summarized in Fig. 1. The characteristics of the included studies are shown in Supplementary Table 1.
Quality of Included Studies and Risk of Publication Bias
There were 12 studies not clearly describing the methods of random sequence generation and/or allocation concealment (selection bias). Two trials showed a high risk of performance and detection bias. Also, risk of performance and detection bias was unclear in four trials, and the risk was judged to be low for the other studies. Two, one, and two studies were considered to have an unclear risk for incomplete outcome data, selective reporting, and other bias, respectively, and the risks were considered low for the others. The risk of bias analysis is summarized in Supplementary Fig. 1.
The funnel plot did not show an asymmetry with the non-significant result of Egger’s test for the outcomes assessed, including change in HbA1c (p = 0.2501), FPG (p = 0.4435), PPG-2h (p = 0.4732), and body weight (p = 0.1022), and OR for incidences of overall hypoglycemia (p = 0.3704), symptomatic hypoglycemia (p = 0.6073), and severe hypoglycemia (p = 0.5744). However, there was evidence of publication bias for change in daily insulin dose (p = 0.0002) and for HbA1c goal attainment (p < 0.0001). The results of the funnel plot analysis are depicted in Supplementary Fig. 2.
Efficacy Outcomes
Change in HbA1c
All enrolled studies involving 6851 patients with T2DM assessed the change in HbA1c levels. The combination therapy of insulin and a DDP4i led to a greater reduction in HbA1c level as compared with the control (WMD = − 0.57%; 95% CI − 0.66, − 0.48; p < 0.0001). A significant heterogeneity among studies was detected (I2 = 82%, p < 0.01). Subgroup analysis showed that control-adjusted reductions in HbA1c from baseline were observed in both the insulin-stable subgroup (WMD = − 0.64%; 95% CI − 0.74, − 0.53) and insulin-flexible subgroup (WMD = − 0.43%; 95% CI − 0.56, − 0.30) (Fig. 2).
Achievement of HbA1c Target Goal
Eighteen studies assessed the proportion of patients achieving the target HbA1c (< 7%). The combination therapy of DPP4i and insulin was associated a higher likelihood of achieving this goal (OR 3.45; 95% CI 2.58, 4.63; p < 0.0001). Significant heterogeneity among studies was detected (I2 = 62%, p < 0.01). In subgroup analysis, the combination therapy of DPP4i and insulin demonstrated a greater chance to achieve the target HbA1c goal in comparison with the control treatment in both the insulin-stable subgroup (OR 4.33; 95% CI 2.90, 6.48) and insulin-flexible subgroup (OR 2.43; 95% CI 1.71, 3.46) (Fig. 3).
Change in FPG
Pooled analysis of 16 studies assessed the change in FPG. The FPG change from baseline was significant between the DPP4i/insulin and control groups (WMD = − 0.53 mmol/L; 95% CI − 0.72, − 0.34; p < 0.0001). The heterogeneity among studies was not significant (I2 = 37%, p = 0.07). Subgroup analysis revealed that the difference in the adjusted change from baseline for the DPP4i/insulin group compared with the control was − 0.64 mmol/L (95% CI − 0.84, − 0.44) in the insulin-stable subgroup and − 0.27 mmol/L (95% CI − 0.66, 0.11) in the insulin-flexible subgroup (Supplementary Fig. 3).
Change in PPG-2h
Seven studies were used for the analysis of the PPG-2h change from baseline, which was significant between DPP4i/insulin and control groups (WMD = − 1.91 mmol/L; 95% CI − 2.24, − 1.58; p < 0.0001). The heterogeneity among studies was not significant (I2 = 5%, p = 0.39). Subgroup analysis showed that the control-adjusted mean change in PPG-2h from baseline was − 1.85 mmol/L (95% CI − 2.18, − 1.53) and − 2.55 mmol/L (95% CI − 3.67, − 1.43) in the insulin-stable and insulin-flexible subgroups, respectively (Supplementary Fig. 4).
Change in Daily Dosage of Insulin Use
For the change in daily insulin dose from baseline, 11 studies were included for the analysis, of which seven studies examined patients on stable insulin dose regimens while the other four studies examined patients with insulin dose titration. DPP4i/insulin treatment led to a greater decrease in daily insulin dose as compared with the control treatment (WMD = − 1.94 IU/day; 95% CI − 2.75, − 1.12; p < 0.0001). The heterogeneity among studies was significant (I2 = 93%, p < 0.01).
In studies adopting a stable insulin dosage, insulin doses were maintained stable throughout the study, except if the insulin dose was adjusted for glycemic rescue. Thus, the adjusted mean change in total daily insulin dose was relatively small (− 1.69 IU/day; 95% CI − 2.87, − 0.51). In contrast, among studies with a titrating insulin dosage, a larger adjusted reduction in daily insulin dose (− 4.70 IU/day; 95% CI − 8.18, − 1.22) was observed, due to a more flexible regimen that allowed up-titration of insulin in the control arm (Supplementary Fig. 5).
Safety Outcomes
Incidence of Hypoglycemia
Pooled analysis of 18 studies with 6209 patients was performed to examine the incidence rate of overall hypoglycemia. Despite the lack of a universal definition for hypoglycemia across the included studies, the risk of developing overall hypoglycemia was similar between the DPP4i/insulin and control groups (OR 0.92; 95% CI 0.76, 1.12; p = 0.4310; significant heterogeneity among studies with I2 = 49%, p < 0.01) (Fig. 4). We also analyzed the risks of symptomatic hypoglycemia and severe hypoglycemia, which did not differ between treatment with DPP4i/insulin and control (symptomatic hypoglycemia, OR 1.08, 95% CI 0.69, 1.68, p = 0.7484; significant heterogeneity among studies with I2 = 79%, p < 0.01; and severe hypoglycemia, OR 1.00, 95% CI 0.66, 1.52, p = 0.9863; non-significant heterogeneity among studies with I2 = 0%, p = 0.98) (Supplementary Figs. 6 and 7).
Among studies with a stable insulin dosage, DPP4i/insulin treatment caused an increased risk of symptomatic hypoglycemia (OR 1.64; 95% CI 1.20, 2.25; p < 0.05) compared with the control. For studies with flexible insulin dosing, DPP4i/insulin did not increase the likelihood of symptomatic hypoglycemia (OR 0.71; 95% CI 0.45, 1.14; p > 0.05) (Supplementary Fig. 6). Irrespective of the subgroup, the risk of developing severe hypoglycemia was not significantly different with DPP4i/insulin relative to the control treatment (Supplementary Fig. 7).
Change in Body Weight
The change in body weight from baseline did not differ significantly between patients receiving DPP4i/insulin and control treatment (WMD = 0.02 kg; 95% CI − 0.30, 0.34; p = 0.8931). The heterogeneity among studies was significant (I2 = 77%, p < 0.01). Subgroup analysis revealed that the adjusted mean change in body weight from baseline was 0.02 kg (95% CI − 0.16, 0.19) in the insulin-stable group and − 0.33 kg (95% CI − 1.51, 0.85) in the insulin-flexible group (Supplementary Fig. 8).
Discussion
The resulting data revealed that the combination therapy of a DPP4i and insulin yields improved glycemic control and decreased daily insulin requirement, without increasing the risk of hypoglycemia and body weight, compared with the insulin control group. This was seen in patients with or without concomitant use of other OADs, such as metformin. Unlike previous meta-analysis, this study evaluated the efficacy and safety of DPP4i in combination with insulin therapy in studies adopting flexible or stable insulin regimens separately. In studies that were designed to flexibly up- or down-titrate the dose of insulin in a fashion mimicking normal clinical practice, the DPP4i and insulin combination treatment provided glycemic benefits and a clear insulin-sparing effect as well as potentially reduced risks of hypoglycemia. These data allowed us to truly evaluate the efficacy and safety of DPP4i as add-on therapy to insulin.
DPP4i have been reported to lower HbA1c levels when used alone and in combination therapy [35, 36]. In a meta-analysis involving 43 RCTs and 19,101 participants, all DPP4i resulted in a greater proportion of patients reaching the HbA1c target of less than 7% than did placebo, regardless of the type of combined antidiabetic drugs [37]. In this study, DPP4i, when added to insulin therapy, led to a greater reduction in HbA1c and achievement of HbA1c target < 7%. Also, the addition of DPP4i to ongoing insulin therapy provided significant reductions in FPG and PPG-2h relative to the control, demonstrating that DPP4i in combination with insulin therapy can improve fasting and postprandial glycemic control. In particular, DPP4i are effective at decreasing postprandial plasma glucose excursion [36, 38], whereas treatment regimens with basal or premixed insulin preparations may not adequately control glycemic excursions in the postprandial state. Therefore, a beneficial effect can be produced by the complementary action of insulin combined with DPP4i.
In most included studies, the insulin dose was designed to remain stable throughout the study period, unless the insulin dose needed to be adjusted because of hypoglycemia or hyperglycemia rescue, which is unlikely to be a true reflection of real-world clinical practice where the clinician would choose between add-on therapy and titration of insulin dosage for patients with poorly controlled T2DM. Therefore, we performed an insulin-flexible subgroup analysis to explore which, insulin up-titration versus addition of a DPP4i, would be more efficacious and safer. DPP4i, when added to insulin titration therapy, led to significantly greater reductions in HbA1c of − 0.43% (− 0.56; − 0.30), FPG of − 0.27 mmol/L (− 0.66; 0.11), and 2-h PPG of − 2.55 mmol/L (95% CI − 3.67, − 1.43) and provided significantly greater odds for achieving the HbA1c target of less than 7% (OR 2.43; 95% CI 1.71, 3.46) relative to the control treatment. Additionally, an adjusted reduction in daily insulin dose (− 4.70 IU/day; 95% CI − 8.18, − 1.22) was observed in the insulin-flexible subgroup. The incidence rates of hypoglycemia varied across the studies included. In this meta-analysis, we observed similar rates of overall hypoglycemia between the DPP4i/insulin and control groups in spite of the greater blood glucose-lowering effect with DPP4i as evidenced by a control-adjusted reduction in HbA1c of 0.57%. These findings are consistent with the known mechanism of action of DPP4i, which enhance insulin secretion by β-cells and/or decrease glucagon release by α-cells in a glucose-dependent manner [6]. Thus, the use of DPP4i in combination with insulin may improve glycemic control without increasing the risk of hypoglycemia.
Several studies evaluated the impact of the addition of a DPP4i to insulin versus active up-titration of insulin on efficacy and safety endpoints. In a study by Hong et al., compared to a 25% increase in insulin dose, adding sitagliptin to an insulin-based regimen was more effective at lowering HbA1c and associated with less hypoglycemia and weight gain over 24 weeks [19]. The similar results were found by Mathieu et al. [27]. In addition, insulin dose titration would likely carry a greater risk of hypoglycemia that should be weighed against any glycemic benefit. In this regard, the benefits of adding a DPP4i to insulin therapy could be even greater when compared to intensified insulin therapy, especially for those particular patients who are concerned about weight gain and/or those more prone to hypoglycemia. In our study, DPP4i/insulin treatment did not increase the likelihood of hypoglycemia in comparison with up-titration of the insulin regime. In fact, the OR values for overall hypoglycemia and symptomatic hypoglycemia were less than 1 in the insulin-flexible group (0.74 and 0.71, respectively), which indicated a decreased likelihood of developing these hypoglycemia events, although the differences were not significant.
This study has several limitations. First, the present meta-analysis comprised studies with various types of insulin regimens and background OADs used, which might introduce some bias. Also, evidence of publication bias for the change in daily insulin dose and for HbA1c goal attainment was observed. The results must therefore be interpreted with caution. Second, algorithms for adjustment of insulin doses were not consistent in the studies included, which might affect the insulin dosage used and the rate of hypoglycemia among studies. Third, the numbers of participants in some treatment groups differed greatly, which might limit the statistical power. For T2DM management, besides glucose management, it is also important to control other cardiovascular risk factors, such as dyslipidemia, excess adiposity, and hypertension.
Conclusion
The findings of the present study indicate that the addition of DPP4i to insulin is associated with a statistically significant reduction in glycemic control as measured by HbA1c, FPG, and PPG-2h, without increasing the risk of hypoglycemia and weigh gain. Similar conclusions were also observed in both stable-dose and flexible-dose insulin subgroups.
References
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843.
Pan C, Yang W, Jia W, Weng J, Tian H. Management of Chinese patients with type 2 diabetes, 1998–2006: the Diabcare-China surveys. Curr Med Res Opin. 2009;25:39–45.
American Diabetes Association. Standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl):S1–212.
McCall AL. Insulin therapy and hypoglycemia. Endocrinol Metab Clin North Am. 2012;41:57–87.
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes Metab. 2007;9:799–812.
Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32:442–58.
Xu W, Mu Y, Zhao J, et al. Efficacy and safety of metformin and sitagliptin based triple antihyperglycemic therapy (STRATEGY): a multicenter, randomized, controlled, non-inferiority clinical trial. Sci China Life Sci. 2017;60:225–38.
Wu D, Li L, Liu C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Obes Metab. 2014;16:30–7.
Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin. 2012;28:513–23.
Chen Y, Liu X, Li Q, et al. Saxagliptin add-on therapy in Chinese patients with type 2 diabetes inadequately controlled by insulin with or without metformin: results from the SUPER study, a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2018;20:1044–9.
Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–55.
Chen C, Yu Q, Zhang S, Yang P, Wang CY. Assessing the efficacy and safety of combined DPP-4 inhibitor and insulin treatment in patients with type 2 diabetes: a meta-analysis. Int J Clin Exp Pathol. 2015;8:14141–50.
Kim YG, Min SH, Hahn S, Oh TJ, Park KS, Cho YM. Efficacy and safety of the addition of a dipeptidyl peptidase-4 inhibitor to insulin therapy in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;116:86–95.
Yang W, Cai X, Gao X, Chen Y, Chen L, Ji L. Addition of dipeptidyl peptidase-4 inhibitors to insulin treatment in type 2 diabetes patients: a meta-analysis. J Diabetes Investig. 2018;9:813–21.
Vos RC, van Avendonk MJ, Jansen H, et al. Insulin monotherapy compared with the addition of oral glucose-lowering agents to insulin for people with type 2 diabetes already on insulin therapy and inadequate glycaemic control. Cochrane Database Syst Rev. 2016;9:CD006992.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.training.cochrane.org/handbook.
Cao Y, Gao F, Zhang Q, et al. Efficacy and safety of coadministration of sitagliptin with insulin glargine in type 2 diabetes. J Diabetes. 2017;9:502–9.
Hirose T, Suzuki M, Tsumiyama I. Efficacy and safety of vildagliptin as an add-on to insulin with or without metformin in japanese patients with type 2 diabetes mellitus: a 12-week, double-blind, randomized study. Diabetes Ther. 2015;6:559–71.
Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795–802.
Kadowaki T, Tajima N, Odawara M, et al. Efficacy and safety of sitagliptin add-on therapy in Japanese patients with type 2 diabetes on insulin monotherapy. Diabetol Int. 2013;4:160–72.
Kadowaki T, Muto S, Ouchi Y, Shimazaki R, Seino Y. Efficacy and safety of saxagliptin in combination with insulin in Japanese patients with type 2 diabetes mellitus: a 16-week double-blind randomized controlled trial with a 36-week open-label extension. Expert Opin Pharmacother. 2017;18:1903–19.
Kadowaki T, Kondo K, Sasaki N, et al. Efficacy and safety of teneligliptin add-on to insulin monotherapy in Japanese patients with type 2 diabetes mellitus: a 16-week, randomized, double-blind, placebo-controlled trial with an open-label period. Expert Opin Pharmacother. 2017;18:1291–300.
Kaku K, Mori M, Kanoo T, Katou M, Seino Y. Efficacy and safety of alogliptin added to insulin in Japanese patients with type 2 diabetes: a randomized, double-blind, 12-week, placebo-controlled trial followed by an open-label, long-term extension phase. Expert Opin Pharmacother. 2014;15:2121–30.
Kanazawa I, Tanaka KI, Notsu M, et al. Long-term efficacy and safety of vildagliptin add-on therapy in type 2 diabetes mellitus with insulin treatment. Diabetes Res Clin Pract. 2017;123:9–17.
Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:252–7.
Linjawi S, Sothiratnam R, Sari R, Andersen H, Hiort LC, Rao P. The study of once- and twice-daily biphasic insulin aspart 30 (BIAsp 30) with sitagliptin, and twice-daily BIAsp 30 without sitagliptin, in patients with type 2 diabetes uncontrolled on sitagliptin and metformin-The Sit2Mix trial. Prim Care Diabetes. 2015;9:370–6.
Mathieu C, Shankar RR, Lorber D, et al. A randomized clinical trial to evaluate the efficacy and safety of co-administration of sitagliptin with intensively titrated insulin glargine. Diabetes Ther. 2015;6:127–42.
Mita T, Katakami N, Shiraiwa T, et al. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the sitagliptin preventive study of intima-media thickness evaluation (SPIKE): a randomized controlled trial. Diabetes Care. 2016;39:455–64.
Ning G, Wang W, Li L, et al. Vildagliptin as add-on therapy to insulin improves glycemic control without increasing risk of hypoglycemia in Asian, predominantly Chinese, patients with type 2 diabetes mellitus. J Diabetes. 2016;8:345–53.
Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11:1145–52.
Sato S, Saisho Y, Kou K, et al. Efficacy and safety of sitagliptin added to insulin in Japanese patients with type 2 diabetes: the EDIT randomized trial. PLoS One. 2015;10:e0121988.
Shankar RR, Bao Y, Han P, et al. Sitagliptin added to stable insulin therapy with or without metformin in Chinese patients with type 2 diabetes. J Diabetes Investig. 2017;8:321–9.
Vilsboll T, Rosenstock J, Yki-Jarvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167–77.
Yki-Jarvinen H, Rosenstock J, Duran-Garcia S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥ 52-week randomized, double-blind study. Diabetes Care. 2013;36:3875–81.
DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649–55.
Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7.
Esposito K, Cozzolino D, Bellastella G, et al. Dipeptidyl peptidase-4 inhibitors and HbA1c target of < 7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2011;13:594–603.
Solis-Herrera C, Triplitt C, Garduno-Garcia Jde J, Adams J, DeFronzo RA, Cersosimo E. Mechanisms of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin when used alone or with metformin in type 2 diabetes: a double-tracer study. Diabetes Care. 2013;36:2756–62.
Acknowledgements
Funding
The study and the journal’s rapid service fee were funded by MSD China.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
AKS and TH performed analyses. RZ collected and assembled the data, wrote sections of the initial draft. JY, QT, YT, AKS, RZ, YZ, SR and TH interpreted the results. All authors conceived and designed research, provided substantive suggestions for revision, reviewed and approved final version of the paper, and for all aspects of the work in ensuring that questions related to the accuracy.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance was provided by Medjaden Bioscience Limited and was funded by MSD China. Shangyu Chai of MSD China Holding Co., Ltd., Shanghai, China, reviewed and provided inputs on drafts of the manuscript. Administrative assistance was provided by Louie Liang of MSD China Holding Co., Ltd., Shanghai, China.
Disclosures
Swapnil Rajpathak, Yuexin Tang and Arvind K. Shah are employees of Merck & Co., Inc., Ruya Zhang and Ye Zhang are employees of MSD China Holding Co., Ltd., Guojuan Chen is ex-employee of MSD China Holding Co., Ltd. Jin Yang, Qing Tian and Tianpei Hong have nothing to disclose.
Compliance with Ethics Guidelines
Ethical approval was unnecessary in this study because it was a systematic review and meta-analysis of existing published articles and therefore does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data relevant to the study are included in the article and uploaded as supplementary information.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12788354.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yang, J., Tian, Q., Tang, Y. et al. Effect of Dipeptidyl Peptidase 4 Inhibitors Used in Combination with Insulin Treatment in Patients with Type 2 Diabetes: A Systematic Review and Meta-analysis. Diabetes Ther 11, 2371–2382 (2020). https://doi.org/10.1007/s13300-020-00914-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00914-x