Abstract
Introduction
We aimed to evaluate the effectiveness of quadruple oral therapy in patients with inadequately controlled type 2 diabetes (T2D) with the use of three types of oral hypoglycemic agents.
Methods
Medical records of 318 patients with T2D who were prescribed quadruple therapy in the Asan Medical Center were reviewed. Changes in glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) levels from baseline were assessed. The regimens of quadruple oral therapy included the following: (1) thiazolidinedione (TZD) add-on to metformin (MET) + sulfonylurea (SU) + dipeptidyl peptidase 4 inhibitor (DPP4i), (2) sodium-glucose cotransporter 2 inhibitor (SGLT2i) add-on to MET + SU + DPP4i, and (3) DPP4i add-on to MET + SU + TZD.
Results
The TZD add-on significantly reduced HbA1c levels by 1.1% (from 9.0 ± 1.1 to 7.9 ± 1.1%, P < 0.001) and FPG levels by 41.4 mg/dL (from 188.9 ± 45.9 to 147.4 ± 51.3 mg/dL, P < 0.001). The SGLT2i add-on changed the mean HbA1c level from 8.9 ± 1.0 to 7.8 ± 1.0%, a reduction of 1.1% (P < 0.001) and changed the mean FPG level from 193.4 ± 46.2 to 152.6 ± 37.0 mg/dL, a reduction of 40.8 mg/dL (P < 0.001). Finally, the DPP4i add-on reduced HbA1c levels by 1.3% (from 9.1 ± 1.3 to 7.8 ± 1.4%, P < 0.001) and FPG levels by 39.3 mg/dL (from 190.7 ± 45.3 to 151.4 ± 41.6 mg/dL, P < 0.001). Patients with higher baseline HbA1c levels (≥ 9.0%) showed a better response to quadruple therapy than those with baseline HbA1c levels lower than 9.0% for all three regimens.
Conclusion
Quadruple oral hypoglycemic therapy can be a feasible option in patients with T2D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Currently, more than five categories of oral hypoglycemic agents are available for the management of type 2 diabetes. |
We aimed to investigate the clinical efficacies of three quadruple combination therapies with oral hypoglycemic agents. |
What was learned from the study? |
Quadruple oral hypoglycemic therapy significantly reduced HbA1c and fasting plasma glucose levels in patients with uncontrolled type 2 diabetes. |
Quadruple oral hypoglycemic therapy is a feasible option for the management of patients with type 2 diabetes who cannot use insulin or refuse injectable therapy. |
Introduction
Type 2 diabetes (T2D) is a progressive disease that is characterized by a deterioration in pancreatic beta cell function and aggravation of insulin resistance [1]. As the disease progresses, several patients with T2D are eventually unable to adequately achieve or maintain glycemic control despite the use of oral hypoglycemic agents [1]. Although insulin therapy has been recommended in patients with T2D showing deteriorating glucose control with the use of oral hypoglycemic agents, these patients often refuse to receive insulin therapy [2]. Common barriers to insulin initiation among patients include patients’ fear of disease progression and needle anxiety [3]. Additionally, weight gain and hypoglycemic episodes are some of the disadvantages of insulin therapy [3].
Currently, several pharmacotherapies are available for glycemic control in T2D. With the development of sulfonylurea (SU) and metformin (MET) in the 1950s, physicians started to prescribe a combination of oral hypoglycemic agents (OHAs) [4, 5]. Since then, several OHAs, including thiazolidinedione (TZD), dipeptidyl peptidase 4 inhibitors (DPP4is), and sodium-glucose cotransporter 2 inhibitors (SGLT2is), have become available [6]. More promisingly, these OHAs target different organs and act via various mechanisms. Therefore, a quadruple combination of OHAs targeting various organs could be a feasible option for patients with poorly controlled T2D. However, despite its potential, only limited data are available on the use of quadruple OHAs [7, 8]. Therefore, the present study investigated the effectiveness of quadruple OHAs in Korean patients with T2D.
Methods
Study Participants
The medical history of all patients treated for T2D at the outpatient clinic of the Department of Endocrinology and Metabolism at the Asan Medical Center (AMC) between January 2012 and November 2017 were reviewed. Patients who had been prescribed a quadruple combination of OHAs from triple OHAs and whose glycated hemoglobin (HbA1c) levels had been followed up were included. Regimens of quadruple oral therapy included the following: (1) TZD add-on to MET + SU + DPP4i, (2) SGLT2i add-on to MET + SU + DPP4i, and (3) DPP4i add-on to MET + SU + TZD. SU included gliclazide and glimepiride. Of the 338 patients with T2D who were selected as study participants for the assessment of changes in HbA1c levels, 20 patients were excluded because their baseline HbA1c levels had not been recorded. Finally, 318 participants were included in the study. The median duration of treatment before a follow-up evaluation was 98 days (interquartile range 91–112 days).
Clinical and Laboratory Measurements
Patient data were collected through a review of electronic medical records. Height and body weight were measured with the participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters. Blood pressure (BP) was measured using an automatic manometer, with the cuff placed on the right arm of the patient after they had been resting for 5 min. Blood samples were drawn from the antecubital vein into vacuum tubes and subsequently analyzed at the certified central laboratory at AMC.
Laboratory serum measurements included the following: HbA1c, fasting plasma glucose (FPG), postprandial 2-h plasma glucose (PP2), fasting C-peptide, fasting insulin, blood urea nitrogen, creatinine, several lipid parameters, and liver enzymes. Ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to measure HbA1c levels. FPG and PP2 levels were measured using the immunoturbidimetric method and the enzymatic colorimetric method using a Toshiba 200-FR auto-analyzer (Toshiba Medical Systems Co., Ltd., Tokyo, Japan), respectively. Serum C-peptide and insulin concentrations were obtained using immunoradiometric assays (TFB, Tokyo, Japan). Total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were measured using enzymatic colorimetry on a Toshiba 200-FR Neo analyzer (Toshiba Medical Systems Co., Ltd., Tokyo, Japan). All enzyme activities were measured at 37 °C. The estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [9].
Outcomes
The primary objective of the study was to determine the efficacy of quadruple regimens by assessing the change in HbA1c levels between baseline and 3 months. The secondary outcome was the percentage of patients who achieved the target of HbA1c < 7.0%. We also assessed the change in BP and in the levels of FPG, PP2, HDL-C, and LDL-C between baseline and 3 months. We evaluated the clinical factors affecting the glycemic response of the patients to quadruple therapy. For this, patients were categorized into groups according to their age, BMI, and baseline HbA1c levels, and the reductions in HbA1c levels were compared. Hypoglycemic events were reviewed on the basis of the patients’ medical records and laboratory data during quadruple treatment.
Statistical Analyses
Continuous variables are expressed as the mean ± standard deviation. Categorical variables are expressed as percentages. One-way analysis of variance with Scheffe’s method was used to assess continuous variables, and the chi-squared test was used to assess categorical variables. A paired t test was performed to assess the changes in HbA1c levels, FPG levels, and body weight from baseline. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software version 20.0 for Windows (SPSS, Inc., Chicago, IL). P < 0.05 was considered statistically significant for all analyses described above.
Ethics Statement
In accordance with the ethical guidelines of the Declaration of Helsinki and Korea Good Clinical Practice, this study was approved by the Institutional Review Board of AMC (2019-0208).
Results
Baseline Characteristics of Study Participants
The characteristics of the patients enrolled in this study are presented in Table 1. The mean age was 59.9 ± 10.2 years, and 57.5% of the participants were male. The mean baseline HbA1c level was 9.0 ± 1.2%. We assessed the baseline characteristics of the study participants according to the quadruple regimen. There were no differences in baseline HbA1c levels among the three groups (P = 0.637). Patients in the SGLT2i add-on group tended to be younger, had a higher BMI, and had better renal function than those in the other groups (P < 0.001, P = 0.018, and P < 0.001, respectively). The TZD add-on group and the SGLT2i add-on group included more women than the DPP4i add-on group (P < 0.001). There were no differences in baseline PP2 levels among the three groups (P = 0.496). Diastolic BP was higher in the SGLT2i add-on group than that in the other two groups (P = 0.001), but the same was not observed for systolic BP (P = 0.496). The TZD add-on and the SGLT2i add-on groups had lower HDL-C levels than the DPP4i add-on group (P = 0.002). Other lipid profiles, including triglycerides and LDL-C, were similar in the three groups. The TZD add-on group and the SGLT2i add-on group showed higher AST and ALT levels than the DPP4i add-on group (P = 0.043 and P = 0.001, respectively).
Efficacy Outcomes According to Quadruple Regimen
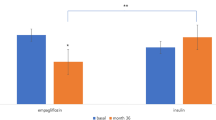
We assessed HbA1c and FPG reductions according to different quadruple regimens (Fig. 1). The TZD add-on reduced HbA1c levels by 1.1% (from 9.0 ± 1.1 to 7.9 ± 1.1%, P < 0.001) and FPG levels by 41.4 mg/dL (from 188.9 ± 45.9 to 147.4 ± 51.3 mg/dL, P < 0.001). In the SGLT2i add-on group, the mean HbA1c level changed from 8.9 ± 1.0 to 7.8 ± 1.0%, with an HbA1c reduction of 1.1% (P < 0.001). The mean FPG level reduced from 193.4 ± 46.2 to 152.6 ± 37.0 mg/dL, showing a reduction of 40.8 mg/dL (P < 0.001). Finally, the DPP4i add-on reduced HbA1c levels by 1.3% (from 9.1 ± 1.3 to 7.8 ± 1.4%, P < 0.001) and FPG levels by 39.3 ± 5.8 mg/dL (from 190.7 ± 45.3 to 151.4 ± 41.6 mg/dL, P < 0.001). There was no significant difference in the glucose-lowering efficacy among the three regimens (P = 0.282 for HbA1c change and P = 0.957 for FPG change). The proportions of patients who achieved the target of HbA1c < 7.0% were 18.7%, 21.3%, and 20.2% in the TZD add-on, SGLT2i add-on, and DPP4i add-on groups, respectively (Fig. 2). There was no significant difference in the proportion of patients achieving the target of HbA1c < 7.0% (P = 0.906).
Changes in glycated hemoglobin (HbA1c) and fasting plasma glucose levels according to different quadruple regimens: a mean (± standard error [SE]) HbA1c (%) levels at baseline and follow-up; b mean (± SE) fasting plasma glucose (mg/dL) levels at baseline and follow-up. *P < 0.05; comparison between baseline and follow-up
All of three regimens were effective in lowering PP2 levels (Supplemental Fig. 1). TZD add-on, SGLT2i add-on, and DPP4i add-on reduced PP2 levels from 274.8 ± 67.9 to 216.4 ± 64.2 mg/dL (P < 0.001), from 334.2 ± 59.6 mg/dL to 239.0 ± 53.6 (P = 0.042), and from 298.5 ± 64.6 to 228.9 ± 63.4 mg/dL (P < 0.001), respectively. There was no significant difference in PP2-lowering effect among the three regimens (P = 0.321). Subsequently, we evaluated the variation of systolic BP, diastolic BP, HDL-C, and LDL-C (Supplemental Fig. 2). HDL-C was increased by TZD add-on (from 43.5 ± 10.5 to 47.3 ± 10.8 mg/dL, P < 0.001) and SGLT2i add-on (from 42.1 ± 9.3 to 44.1 ± 9.5 mg/dL, P = 0.041). Systolic BP, diastolic BP, and LDL-C did not change significantly after the fourth OHA add-on in all groups.
Body Weight Changes and Hypoglycemic Events
We evaluated body weight changes in patients taking different quadruple regimens (Fig. 3). The TZD add-on increased body weight by 1.2 kg (from 68.3 ± 12.3 to 69.5 ± 12.5 kg, P < 0.001). In the SGLT2i add-on group, the mean body weight reduced from 75.2 ± 13.3 to 73.1 ± 12.6 kg, showing a weight reduction of 2.1 kg (P < 0.001). Finally, the DPP4i add-on did not change body weight significantly (from 72.0 ± 12.0 to 72.5 ± 12.2 kg, P = 0.111). The weight-lowering effect of SGLT2i add-on was significant when compared with TZD or DPP4i (P < 0.001 for both comparisons). Body weight changes by TZD add-on or DPP4i add-on were not statistically different (P = 0.097).
On the basis of self-report, a total of 23 hypoglycemic events (7.2% of the total patients) were observed: 18 cases (9.6%) in the TZD add-on group, three cases (6.4%) in the SGLT2i add-on group, and two cases (2.4%) in the DPP4i add-on group. The incidence of hypoglycemia did not show a significant difference among the groups (P = 0.101). However, almost all hypoglycemic events were mild and self-limiting, except in one case. One patient was admitted because of symptomatic hypoglycemia with a self-monitored blood glucose level of 80 mg/dL after taking the quadruple oral therapy (TZD add-on).
Clinical Parameters Affecting Glucose-Lowering Effect of Quadruple Regimens
Subgroup analyses were performed to elucidate the clinical factors affecting the glucose-reducing effect of quadruple regimens (Fig. 4). No significant differences were observed when the patients were grouped by age or BMI (Fig. 4a–f). However, in a subsequent analysis based on baseline HbA1c levels, patients with higher baseline HbA1c levels exhibited a more significant reduction in HbA1c levels during follow-up examinations than those with lower baseline HbA1c levels (Fig. 4g–i). This trend was observed consistently in all three regimens (i.e., TZD add-on, SGLT2i add-on, and DPP4i add-on).
Discussion
Our results showed that quadruple oral therapy was effective and safe in patients with T2D. In real-world clinical practice, a considerable number of patients are prescribed with quadruple oral therapy. In our study, the regimen of quadruple therapy was variable. Usually, the patients were already receiving MET and SU; following these two most commonly used OHAs, TZD, DPP4i, and/or SGLT2i were added according to the patient’s clinical status. SGLT2is tended to be prescribed to young patients, obese patients, and patients with preserved renal function. TZD or SGLT2i was commonly added as the fourth OHA for female patients and patients with low HDL levels and high liver enzymes. Regardless of the regimen, addition of the fourth OHA was effective in lowering HbA1c, FPG, and PP2 levels, and a higher baseline HbA1c level predicted a better glycemic response.
While OHAs may initially help in achieving glycemic control, the progressive nature of T2D makes it difficult for the patients to maintain favorable glycemic control [10, 11]. The American Diabetes Association (ADA) has recommended injectable therapies, including glucagon-like peptide 1 receptor agonist (GLP-1 RA) and insulin, if the HbA1c level exceeds the target despite administration of dual or triple oral therapy [6]. However, refusal to inject oneself and the fear of weight gain or hypoglycemia may hinder compliance to insulin therapy [12]. Although GLP-1 RA is more convenient and has a lower risk of hypoglycemia than insulin, patients still experience inconvenience upon administration as it is an injectable therapy [13]. Currently, several pharmacotherapies are available for glycemic control in T2D, and these agents act via different mechanisms. In detail, the predominant effect of MET is the inhibition of hepatic glucose production, whereas TZD mainly acts by improving peripheral uptake and utilizing glucose in the muscle and fat, finally decreasing liver glucose production [14, 15]. SU works by stimulating insulin release from insulin-secreting beta cells [16], and DPP4is mimic the effects of incretin hormones, including the stimulation of insulin secretion and inhibition of glucagon secretion [15, 17]. The most novel drugs, SGLT2is, inhibit the reabsorption of glucose in the kidney and, therefore, lower blood sugar levels [18]. As each OHA has its unique mechanism, the combination of these drugs could be a feasible therapeutic strategy for T2D.
In the present study, we found that physicians are, not infrequently, prescribing quadruple combinations in real-world clinical practice. In the current guidelines [6, 19], MET is the preferred initial pharmacologic agent administered for the treatment of T2D. Our data showed that in our study population, the most frequently used dual combination therapy was MET plus SU. In Korea, SU is frequently used as the second OHA because of its low cost and high efficacy [20]. After MET and SU combination, TZD, a DPP4i, and/or an SGLT2i were added. SGLT2is are well known to reduce body weight [21], and our data also demonstrated significant weight loss by the SGLT2i add-on. However, the TZD add-on increased body weight, and the DPP4i add-on did not change body weight. Considering these results, an SGLT2i was frequently added to the regimens of obese patients in our study. Additionally, it has been reported that the efficacy of SGLT2is is greater in patients with preserved renal function than in those without preserved renal function [22, 23]. In our study, the SGLT2i add-on group demonstrated higher eGFR than the other two groups. These prescribing patterns show that physicians select the added OHAs on the basis of the characteristics of the agents.
Our data also demonstrated that the addition of the fourth OHA showed favorable glucose-lowering effect regardless of the regimen. Before the addition of the fourth OHA, the mean HbA1c level in the total population was 9.0 ± 1.2%. The addition of the fourth OHA reduced the HbA1c level to 7.9 ± 1.2%, showing a 1.1% reduction in HbA1c levels. Subgroup analyses revealed that patients with higher baseline HbA1c levels showed a more significant reduction in HbA1c levels than those with lower baseline HbA1c levels. Although a reasonable target for several adults is HbA1c < 7%, which was achieved in only approximately one-fifth of the patients in our study, less stringent HbA1c goals, such as a target of HbA1c < 8%, may be appropriate for patients with comorbid conditions or a history of severe hypoglycemia according to the ADA guidelines [24]. Furthermore, regarding hypoglycemia, almost all hypoglycemic events were mild and self-limiting. Only one patient was admitted to hospital because of hypoglycemic symptoms. The overall incidence of hypoglycemic events was low, and only 7.2% of the total patients experienced hypoglycemia. Therefore, quadruple oral therapies could be a feasible therapeutic option in patients with poor glycemic control and less stringent HbA1c goals, especially when the patients refuse injection therapies.
In the present study, we evaluated the variation of BP, HDL-C, and LDL-C (Supplemental Fig. 2). SGLT2i is well known for its BP-lowering effect [25]; however, in this study, the BP reduction was not statistically significant (from 132.8 ± 17.8 to 126.8 ± 23.5 mmHg, P = 0.088 for systolic BP and from 78.0 ± 13.3 to 75.6 ± 10.7 mmHg, P = 0.176 for diastolic BP), possibly owing to the small number of participants. A previous summary analysis of double-blind, placebo-controlled studies with TZD reported that pioglitazone increased HDL-C levels and did not significantly affect LDL-C levels, whereas rosiglitazone increased both HDL-C and LDL-C levels in patients with T2D [26]. A multicenter randomized controlled trial reported that lobeglitazone improved HDL-C levels and did not increase LDL-C levels in patients with T2D [27]. We observed that the TZD add-on group, in which pioglitazone or lobeglitazone was added, had a significant improvement in HDL-C levels, but LDL-C levels remained unchanged; these results are consistent with the previous reports. SGLT2i has been known to increase HDL-C levels in patients with T2D [28, 29], and our results are consistent with these studies.
This study has several limitations. First, this was a retrospective study based on reviews of medical records and conducted in a single center with a relatively small number of participants, potentially limiting the generalizability of the results. Considering that there was no placebo group or no insulin-treated group in this study, we could not compare the effectiveness and safety of the quadruple oral therapies with those of placebo or insulin therapy. Second, the follow-up period was relatively short; therefore, we could not assess the long-term effects of quadruple oral therapies. Finally, considering that we reviewed the medical records of patients to evaluate the hypoglycemic events and that several of the patients did not record their self-monitored blood glucose levels when they experienced hypoglycemic symptoms, we could not use the conservative definition of hypoglycemia based on a documented capillary blood glucose value of 70 mg/dL. In addition, the reports on the hypoglycemic episodes are not reliable because they are based on self-reporting and some episodes may have been missed.
Although the retrospective and observational nature of our study is a limitation, it has the advantage of reflecting the current state of T2D management in the real world. Although there have been a few studies on quadruple therapy in T2D, these studies focused on the effectiveness of SGLT2i-based quadruple regimen in patients with poorly controlled T2D and did not consider variable combinations of quadruple OHA therapies [7, 8]. In addition, the major strength of our study was contributing additional evidence for recommendation or consideration of quadruple therapy for some patients in the future. Despite the fact that the quadruple oral therapy is widely used in clinical practice, the guidelines do not suggest nor recommend quadruple therapy yet, limiting the use of this therapeutic option. Therefore, the results of this study are of clinical importance and could be the first step in suggesting variable quadruple oral therapies as feasible options for management of T2D. Considering the aforementioned limitations, it is necessary that larger prospective studies support the results of our study, and the use of quadruple oral therapies for specific populations of patients with T2D and suboptimal glycemic control.
Conclusion
Quadruple oral therapy can be a viable medical strategy for patients with T2D who show inadequate glucose control with the use of three OHAs.
References
Lingvay I, Legendre JL, Kaloyanova PF, Zhang S, Adams-Huet B, Raskin P. Insulin-based versus triple oral therapy for newly diagnosed type 2 diabetes: which is better? Diabetes Care. 2009;32:1789–95.
Barnett AH, Dreyer M, Lange P, Serdarevic-Pehar M. An open, randomized, parallel-group study to compare the efficacy and safety profile of inhaled human insulin (Exubera) with glibenclamide as adjunctive therapy in patients with type 2 diabetes poorly controlled on metformin. Diabetes Care. 2006;29:1818–25.
Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18–24.
Quianzon CC, Cheikh IE. History of current non-insulin medications for diabetes mellitus. J Community Hosp Intern Med Perspect. 2012;2. https://doi.org/10.3402/jchimp.v2i3.19081.
Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60:1566–76.
American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;2019(42):S90–S102.
Ku EJ, Lee DH, Jeon HJ, Oh TK. Effectiveness and safety of empagliflozin-based quadruple therapy compared with insulin glargine-based therapy in patients with inadequately controlled type 2 diabetes: an observational study in clinical practice. Diabetes Obes Metab. 2019;21:173–7.
Ku EJ, Lee DH, Jeon HJ, Oh TK. Empagliflozin versus dapagliflozin in patients with type 2 diabetes inadequately controlled with metformin, glimepiride and dipeptidyl peptide 4 inhibitors: a 52-week prospective observational study. Diabetes Res Clin Pract. 2019;151:65–73.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Andayani TM, Mohamed Ibrahim MI, Asdie AH. Comparison of the glycemic control of insulin and triple oral therapy in type 2 diabetes mellitus. J Diabetes Endicronol. 2010;1:13–8.
Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000.
Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–9.
Sikirica MV, Martin AA, Wood R, Leith A, Piercy J, Higgins V. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403–12.
Petersen KF, Krssak M, Inzucchi S, Cline GW, Dufour S, Shulman GI. Mechanism of troglitazone action in type 2 diabetes. Diabetes. 2000;49:827–31.
Lorenzati B, Zucco C, Miglietta S, Lamberti F, Bruno G. Oral hypoglycemic drugs: pathophysiological basis of their mechanism of actionoral hypoglycemic drugs. Pharmaceuticals (Basel). 2010;3:3005–200.
Aguilar-Bryan L, Nichols CG, Wechsler SW, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–6.
Knop FK, Vilsboll T, Holst JJ. Incretin-based therapy of type 2 diabetes mellitus. Curr Protein Pept Sci. 2009;10:46–55.
Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5:355–66.
Korean Diabetes Association. Treatment guideline for diabetes. Seoul: Korean Diabetes Association; 2019.
Korean Diabetes Association. Diabetes fact sheet in Korea 2018. Seoul: Korean Diabetes Association; 2018.
Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs. 2019;79:219–30.
Lee JY, Kim G, Kim SR, et al. Clinical parameters affecting dapagliflozin response in patients with type 2 diabetes. Diabetes Metab. 2017;43:191–4.
Yagi S, Aihara KI, Kondo T, et al. Predictors for the treatment effect of sodium glucose co-transporter 2 inhibitors in patients with type 2 diabetes mellitus. Adv Ther. 2018;35:124–34.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S61–S70.
Yaribeygi H, Atkin SL, Sahebkar A. Mechanistic effects of SGLT2 inhibition on blood pressure in diabetes. Diabetes Metab Syndr. 2019;13:1679–83.
van Wijk JP, de Koning EJ, Martens EP, Rabelink TJ. Thiazolidinediones and blood lipids in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2003;23:1744–9.
Kim SG, Kim DM, Woo JT, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014;9:e92843.
Cha SA, Park YM, Yun JS, et al. A comparison of effects of DPP-4 inhibitor and SGLT2 inhibitor on lipid profile in patients with type 2 diabetes. Lipids Health Dis. 2017;16:58.
Fadini GP, Bonora BM, Zatti G, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol. 2017;16:42.
Acknowledgements
Funding
No funding or sponsorship was received for this study or the publication of this article. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Author Contributions
WJL conceived this study. YKC and WJL contributed to the design of the study. YKC, JL, HSK, and CHJ conducted data collection. YKC performed the analysis. JYP, CHJ, and WJL interpreted the results. YKC wrote the initial draft of the manuscript, with revisions performed by all authors. The final manuscript was approved by all authors. YKC and WJL are the guarantors of this work.
Disclosures
Yun Kyung Cho, Jiwoo Lee, Hwi Seung Kim, Chang Hee Jung, Joong-Yeol Park, and Woo Je Lee have nothing to disclose.
Compliance with Ethics Guidelines
In accordance with the ethical guidelines of the Declaration of Helsinki and Korea Good Clinical Practice, this study was approved by the Institutional Review Board of AMC (2019-0208).
Data Availability
All data generated or analyzed during this study are included in this published article as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12630140.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cho, Y.K., Lee, J., Kim, H.S. et al. Clinical Efficacy of Quadruple Oral Therapy for Type 2 Diabetes in Real-World Practice: A Retrospective Observational Study. Diabetes Ther 11, 2029–2039 (2020). https://doi.org/10.1007/s13300-020-00881-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00881-3