Abstract
Introduction
Teneligliptin is an antidiabetic medication that has been approved for the management of type 2 diabetes mellitus (T2DM) in Japan, South Korea and India. It is one of the most commonly prescribed antihyperglycaemic agents. The aim of this study was to assess the effectiveness of teneligliptin in improving glycemic control amongst Indian patients with T2DM in a real-world setting.
Methods
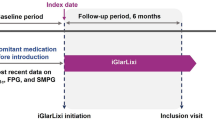
This was a retrospective observational study in which a predesigned structured proforma was used to collect information from hospital records of 18 medical centres across India. All participating centres were established primary care hospitals with adequate record keeping, a pre-determined condition in the study design. Data were collected during the period of January 2019 to June 2019. Data extracted from patient records, including glycaemic parameters, concomitant drugs, drug dosage and duration, were collated. The effectiveness of teneligliptin was assessed by analyzing the mean change in glycosylated haemoglobin (HbA1c), fasting plasma glucose (FPG) and post-prandial plasma glucose (PPG) at 12 weeks after initiation of teneligliptin.
Results
Data from 10,623 patients were available for analysis. The mean age of the enrolled patients was 51.86 ± 11.76 years. At 12 weeks after initiation of teneligliptin as monotherapy or add-on to other medications (combination therapy), the patients showed a signficant decrease from baseline in mean HbA1c, FPG and PPG. Mean HbA1c dropped from 8.66 ± 1.15% at baseline to 7.67 ± 1.28% at 12 weeks (71 ± 12.6 to 60 ± 14 mmol/mol), with a difference of − 0.99% (95% confidence interval [CI] 0.96–1.02) or − 10.8 (95% CI 10.5–11.1) mmol/mol (p < 0.0001). The mean reductions in FPG and PPG were 43.12 mg/dL (2.39 mmol/L) and 87.73 mg/dL (4.87 mmol/L) (both p < 0.0001) respectively. HbA1c (%) reductions with teneligliptin when used as add-on to metformin, add-on to metformin + sulfonylurea combination and add-on to metformin + sulfonylurea + alpha glucosidase inhibitor combination were 0.76% (8.3 mmol/mol), 1.24% (13.6 mmol/mol) and 1.04% (11.4 mmol/mol), respectively. Teneligliptin also significantly reduced HbA1c (1.13% or 12.4 mmol/mol, p < 0.0001) in patients with impaired renal function, without worsening the estimated glomerular filtration rate. Teneligliptin consistently reduced HbA1c across all three age categories tested—by 1% (10.9 mmol/mol) in patients aged < 60 years, by 1.15% (12.6 mmol/mol) in patients aged 60–75 years and by 0.88% (9.6 mmol/mol) in patients aged > 75 years.
Conclusion
Teneligliptin significantly improved glycaemic parameters in Indian patients with T2DM when prescribed either as monotherapy or as an add-on to one or more other commonly prescribed antihyperglycaemic agents.
Similar content being viewed by others
There are a limited number of drug utilization studies of teneligliptin in real-world settings despite this medication being a commonly prescribed gliptin in India for the mangement of type 2 diabetes mellitus (T2DM). |
We collected and analysed the data of > 10,000 patients for changes in the glycemic parameters following treatment with teneligliptin as monotherapy and as add-on with other oral antihyperglycemic agents (combination therapy). |
The effectiveness of teneligliptin was evaluated as monotherapy and in combination therapy in the whole patient population, as well as in sub-analyses according to age, renal impairment and insulin therapy. |
Teneligliptin, either as monotherapy or as an add-on therapy, significantly improved glycaemic parameters, leading to the conclusion that it can be considered as an effective add-on antidiabetic agent in the management of Indian patients with T2DM. |
Although a cause-and-effect relationship cannot be determined using data from retrospective studies due to limited access, our results are useful by providing preliminary data and guiding the development of future prospective long-term studies. |
Introduction
Type 2 diabetes mellitus (T2DM) is a global pandemic, and India is steadily emerging as the diabetes capital of world [1]. The number of patients with diabetes in India is likely to reach 134.2 million by 2045 [2]. This relatively recent increase in prevalence of T2DM can be attributed to multiple factors [3].
Management of T2DM entails lifestyle changes and pharmacological therapy, with the latter ideally meeting the criteria of being efficacious, durable, safe and cost effective. Dipeptidyl peptidase-4 inhibitors (DPP-4Is; also known as “gliptins”) are a relatively novel class of antihyperglycaemic agents widely used in the management of T2DM [4]. Although the mechanism of action of all DPP-4Is is same, individual gliptins may differ from each other in terms of their pharmacokinetics, pharmacodynamics and potency [5].
Teneligliptin is a class 3 DPP4I with a unique structure [6] that enhances its potency and selectivity, such that it has a fivefold higher activity than other gliptins and produces more extensive inhibition of DPP-4 [7]. It has been available in India since 2015 and is widely used both as monotherapy and in combination with other medications. In addition to its high potency and selectivity, teneligliptin is available at an affordable cost and, consequently, it is very commonly prescribed in India. At the time of initiating the study reported here, teneligliptin was the cheapest gliptin available in India.
Drug utilization studies of teneligliptin in real-world settings are limited despite it being a commonly prescribed gliptin in India. While studies in India have reported real-world evidence (RWE) of teneligliptin, they are not as extensive as the analysis of data from the case report forms (CRFs) of the post-marketing surveillance (RUBY surveillance) conducted by Kadowaki et al. in patients with T2DM in a large Japanese population [8]. Such large-scale studies are needed to understand the factors influencing the management of diabetes in the real-world setting.
The study reported here was therefore designed to evaluate the effectiveness of teneligliptin in the Indian population in the real-world setting.
Methods
This was a retrospective audit/analysis in which a predesigned CRF was used to collate patient information from hospital/clinic records of 18 centres across India. All participating centres were established primary care hospitals which maintained an adequate level of record keeping as assessed by pre-determined criteria in the study design. Data were collected from January 2019 to June 2019. Patients with T2DM who were aged ≥ 18 years and had been treated with teneligliptin for a minimum of 12 weeks (either as monotherapy or in combination with other antidiabetic drugs) were eligible to participate in the study. Patients were excluded if there was any modification in the treatment regimen of other antidiabetic drugs during the 12-week period. Patient details extracted from the medical records included age, gender, concomitant drug or medical history, glycaemic parameters, drug dosage and duration of diabetes. Information was collected either from a hard copy or soft copy of the medical record, and data were collated on CRFs for this study (denoted the TREAT-INDIA 2 study) and analysed further. The effectiveness of teneligliptin was analysed on the basis of mean change in glycaemic parameters, including glycosylated haemoglobin (HbA1c), fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) in the 3-month treatment period. Data were compiled on Microsoft Excel (Microsoft Corp., Redmond, WA, USA) and analysed using SPSS software version 15 (IBM Corp., Armonk, NY, USA). Categorical data were represented in simple percentage and mean ± standard deviation. The paired t test was used to compare change in the glycemic parameters. A p value < 0.05 was considered to be significant.
Prior to collecting patient information approval was obtained from the Institutional Ethics Committee (Suraksha Ethics committee. Reg No: ECR/644/Inst/MH/2014/RR-17). The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Results
Data from a total of 10,623 patients from 18 hospitals/clinics were analysed; of these 18 participating centres, seven were located in southern India, three were located in eastern India and four each were located in northern and western India. Table 1 shows the baseline demographic and clinical characteristics of the patients. The average age of patients was 51.86 ± 11.76 years, and the male to female ratio was 1.1:1. The duration of T2DM (four categories of duration) and percentage of patients in each duration category were: < 5 years (53% of patients); 5–10 years (33%); 11–15 years (11%); > 15 years (3%). Hypertension (56% of patients) was the most common comorbidity, followed by dyslipidemia (25%), chronic kidney disease (8%), coronary artery disease (6%), chronic liver disease (4%) and stroke (1%).
There was a statistically significant (all p < 0.0001) improvement from baseline in mean HbA1c (− 0.99%, 95% confidence interval [CI] 0.96–1.02 or − 10.8, [95% CI 10.5–11.1] mmol/mol), FPG (− 43.12 mg/dL, 95% CI 42.5–43.7 or – 2.39 [95% CI 2.36–2.43] mmol/L) and PPG (− 87.73 mg/dL, 95% CI 86.5—88.9 or – 4.87 [95% CI 4.8–4.93] mmol/L) at the end of 12 weeks of teneligliptin therapy in the total patient population (Table 2). The glycaemic target of HbA1c ≤ 7%, FPG ≤ 130 mg/dL and PPG ≤ 180 mg/dL was achieved by 35, 81 and 71% of patients, respectively, after 12 weeks of teneligliptin treatment (Table 3). Teneligliptin was prescribed as a monotherapy in 4% of patients and as a part of add-on therapy in 96% patients (Table 1). Of the combination therapies, teneligliptin with metformin was the most common combination prescribed (40%), followed by teneligliptin with metformin and sulfonylureas (SUs), prescribed in 34% patients (Table 1).
Table 4 depicts the change in HbA1c in patients on the different treatment regimens based on baseline HbA1c; it was observed that reductions in HbA1c were greater in patients having higher baseline HbA1c levels. HbA1c (%) reduction with teneligliptin when used as monotherapy, as add-on to metformin therapy, as add-on to metformin + SUs and as add-on to metformin + SUs + alpha glucosidase inhibitor (AGI) combination was 0.70% (7.7 mmol/mol), 0.76% (8.3 mmol/mol), 1.24% (13.6 mmol/mol) and 1.04% (11.4 mmol/mol), respectively. Teneligliptin used in conjunction with insulin, with or without other oral antidiabetic drugs (OADs), reduced HbA1c by 1.48% (95% CI 1.21–1.74) or by 16.2 mmol/mol (95% CI 13.2–19.0) (p < 0.0001. There was a statistically significant reduction in all the glycemic parameters with teneligliptin monotherapy and in all combinations of teneligliptin and antidiabetic drugs (Table 5).
The data were subjected to sub-analysis to assess the effectiveness of teneligliptin in specific patient populations, such as patients with impaired renal function (defined as estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73m2; n = 549). In these patients with impaired renal function who were prescribed teneligliptin, mean changes in HbA1c, FPG, and PPG were − 1.13% (95% CI 1.03–1.22) or − 12.4 (95% CI 11.3–13.3) mmol/mol (p < 0.0001), − 58.71 mg/dL (95% CI 57.2–60.18) or − 3.26 (95% CI 3.17–3.34) mmol/L (p < 0.0001) and − 92.74 mg/dL (95% CI 88.85–96.6) or − 5.15 (CI 4.93–5.36) mmol/L (p < 0.0001,) respectively (Table 6). No significant changes from baseline in eGFR were observed at 12 weeks after initiating teneligliptin therapy.
Data were also analysed to assess the effectiveness of teneligliptin in various age group categories (< 60 years [n = 7593], 60–75 years [n = 2872], > 75 years [n = 158]). It was observed that after initiation of teneligliptin, there was a significant reduction in all the glycaemic parameters and that this reduction was consistent in all age groups. The reductions in HbA1c in patients aged < 60, 60–75, and > 75 years were 1% (95% CI 0.96–1.03) or 10.9 mmol/mol (95% CI 10.5–11.3) (p < 0.0001), 1.15% (95% CI 0.88–1.01) or 12.6 (95% CI 9.6–11.0) mmol/mol (p < 0.0001) and 0.88% (95% CI 0.58–1.17) or 9.6 (CI 6.3–12.8) mmol/mol (p < 0.0001), respectively (Table 7).
Discussion
Real-world data regarding the efficacy of teneligliptin in Indian patients with T2DM are limited. To the best of our knowledge, our study is the largest analysis of real-world data on the use of teneligliptin not just in India, but worldwide, with data of 10,623 patients used to analyse the effectiveness of teneligliptin on various blood glucose parameters. The only other large-scale study conducted to date was a Japanese study involving 10,532 patients [8]. Our data demonstrate the effectiveness of teneligliptin to improve glycaemic control in Indian patients with T2DM. Statistically significant (p < 0.0001) improvements in glycemic parameters were observed not only in the overall patient population but also in patients with renal impairment as well as in those in different age groups (i.e. aged < 60 years, 60–75 years and > 75 years).
DPP-4Is, also known as gliptins, are a relatively new class of antidiabetic agents that have revolutionized the management of T2DM [4]. DPP-4Is are usually prescribed in patients who have not responded well to other antidiabetic drugs such as metformin and SUs. In our study, teneligliptin was preferred as an add-on therapy (96%)—rather than as monotherapy—in patients who had not responded to primary therapy of metformin and/or SUs. This prescription pattern of teneligliptin was also highlighted in a study conducted by Kumar et al. [9], who observed that teneligliptin was preferred as an add-on therapy to metformin and SU. In our patient population, teneligliptin was added to first-line metformin in 40% of patients and to the metformin + SU combination in 34% of patients. The metformin + SU combination remains the mainstay first-line diabetes therapy in India, probably due to its cost effectiveness. However, the natural history of T2DM warrants the use of additional pharmacological agents [10, 11]. Teneligliptin, a relatively new DPP-4I, offers the convenience of once-daily dosing, weight neutrality and a lower risk of hypoglycaemia; consequently, it is commonly used as an add-on to metformin and/or SUs [12].
The effectiveness of teneligliptin in India was first highlighted in the RWE study TREAT-INDIA (n = 4305) conducted by Ghosh et al. [13] in which teneligliptin as a monotherapy and add-on therapy was shown to significantly reduce HbA1c (overall reduction 1.37 ± 1.15% or 15.0 ± 12.6 mmol/mol), FPG (overall reduction 51.29 ± 35.41 mg/dL or 2.85 ± 1.97 mmol/L) and PPG (overall reduction 80.89 ± 54.27 mg/dL or 4.49 ± 3.01 mmol/L). In the present analysis, denoted the TREAT-INDIA 2 study, our patient population was large (n = 10,623) and comprised patients from different regions of country. We also observed that at 12 weeks after initiation of teneligliptin therapy there was a significant decrease from baseline in FPG (43.12 mg/dL or 2.39 mmol/L), PPG (87.73 mg/dL or 4.87 mmol/L) and HbA1c (0.99% or 10.8 mmol/mol). An important difference between the TREAT INDIA 1 and 2 studies is that the TREAT INDIA 2 study also provides insights on the effectiveness of teneligliptin in specific patient populations, such as patients with T2DM with renal impairment and patients with T2DM of different ages. The findings of these above two large-scale RWE studies in the Indian population reflect the effectiveness of teneligliptin. Similar findings were reported in another real-world efficacy study conducted in India by Nashikkar et al. [14]. Changes in HbA1c, FPG and PPG from baseline to end of the study (after 8 weeks of teneligliptin therapy) were − 1.22 ± 1.12% or − 13.3 ± 12.2 mmol/mol (p = 0.001), − 35.8 ± 25.5 mg/dL or – 1.99 ± 1.42 mmol/L (p = 0.001) and − 60.7 ± 28.6 mg/dL or – 3.37 ± 1.59 mmol/L (p = 0.001), respectively. The effectiveness of teneligliptin was also highlighted by Li et al. [15] in a systematic meta-analysis of ten randomized controlled trials (RCTs) in which teneligliptin significantly (p < 0.00001) decreased HbA1c (0.82% or 9.0 mmol/mol), FPG (18.32 mg/dL or 1.02 mmol/L) and PPG (46.94 mg/dL or 2.61 mmol/L). These data lead to the conclusion that teneligliptin effectively improves glycaemic control and is useful in the management of hyperglycaemia in patients with T2DM.
Subgroup analysis of our study population highlights the difference in the reduction in glycemic parameters obtained with teneligliptin as monotherapy and as add-on therapy. HbA1c reduction was 0.70% (7.7 mmol/mol) in patients on teneligliptin monotherapy; in comparison, in patients on combination therapy with teneligliptin as add-on, HbA1c reduction was 0.76% (8.3 mmol/mol) in patients receiving teneligliptin + metformin combination therapy (two drugs), 1.24% (13.6 mmol/mol) in patients receiving metformin + SU combination therapy (three drugs) and 1.04% (11.4 mmol/mol) in patients receiving metformin + SUs + AGIs combination therapy (four drugs). Similar results were seen with the other glycaemic parameters FPG and PPG. These results were also highlighted in the TREAT-INDIA study [13] which reported that HbA1c was reduced by 0.98% (10.7 mmol/mol) with teneligliptin as monotherapy and by 1.11% (12.1 mmol/mol), 1.51% (16.5 mmol/mol), 1.62% (17.7 mmol/mol) and 1.65% (18.0 mmol/mol) when teneligliptin was part of combination therapies consisting of two, three, four and five drugs, respectively. The above results also highlight the importance of adding and continuing teneligliptin as a part of a combination therapy regimen.
Teneligliptin as monotherapy was also effective in this subset of patients in our study (n = 390), significantly (p < 0.001) reducing HbA1c (0.70% or 7.7 mmol/mol), FPG (39.87 mg/dL or 2.21 mmol/L) and PPG (48.30 mg/dL or 2.68 mmol/L). Although, metformin with lifestyle modification is considered to be the initial line of therapy [16], in conditions which prevent the use of metformin, such as patients with renal impairment, the role of metformin is questionable [17] and thus the use of other drugs is justifiable. Kutoha et al. [18] evaluated the use of teneligliptin as first-line therapy in patients newly diagnosed with T2DM. The results of that study showed that teneligliptin significantly reduced HbA1c from 10.34 ± 2.06% (89 ± 22.5 mmol/mol) at baseline to 8.38 ± 2.23% (68 ± 24.4 mmol/mol) at 3 months (p < 0.00001), and FPG from 211.3 ± 68.4 mg/dL (11.73 ± 3.8 mmol/L) at baseline to 167.3 ± 70.2 mg/dL (9.29 ± 3.9 mmol/L) at 3 months (p < 0.0002). Raghavan et al. [19] conducted a 12-week randomized trial in which they compared the effectiveness of teneligliptin with metformin in patients newly diagnosed with T2DM. The results showed that teneligliptin and metformin had similar efficacy in reducing mean HbA1c (0.60% [6.6 mmol/mol] vs. 0.52% [5.7 mmol/mol], respectively), FPG (19.4 mg/dl [1.08 mmol/L] vs. 16.2 mg/dl [0.9 mmol/L], respectively) and PPG (49.8 mg/dl [2.76 mmol/L] vs. 36.8 mg/dl [2.04 mmol/L], respectively). Agarwal et al. [20] reported a significant decrease in mean HbA1c (least squares means difference = − 0.31 ± 1.25 % [− 3.4 ± 13.7 mmol/mol], p = 0.0037) in a 16-week, multicentre, double-blind, placebo-controlled phase 3 trial in India, with a higher percentage (43.6%) of patients achieving the target HbA1c of < 7% with teneligliptin.
When used as an add-on therapy, we found that teneligliptin was added to almost all classes of antidiabetic medications prescribed currently. In our study population, teneligliptin was also added to insulin and other OADs. Insulin therapy is considered to be the most potent hypoglycemic therapy for patients with uncontrolled T2DM, but increasing the insulin dosage is associated with increased risk of hypoglycaemia and weight gain [21,22,23]. Teneligliptin, when added to insulin therapy, has been shown to decrease the fluctuation in blood glucose levels and increase the proportion of time in the normal glucose range compared to time in hyperglycemia over a 24-h period [24]. Such fluctuations are an important triggering factor for oxidative stress and cardiovascular diseases [25]. Kadowaki et al. [26] in their 16-week RCT reported a significant decrease in blood glucose parameters when teneligliptin was added to insulin. Our results with teneligliptin were similar to those observed in these previous studies.
In our study the combination of teneligliptin and pioglitazone was prescribed in only one patient. AGIs, due to their complementary and different mechanism of action to teneligliptin, increase glucagon-like peptide-1 activity and may provide a valuable option to patients [27,28,29]. In our study, the therapeutic combination of AGI + teneligliptin was used along with other OADs and achieved a significant decrease in glycemic parameters. Several trials have evaluated the efficacy and safety of gliptins with SUs and have reported a significant improvement in glycemic control [30]. Sodium glucose co-transporter 2 (SGLT2) inhibitors are relatively new class of drugs in the diabetes management algorithm. The combination of teneligliptin with SGLT2 inhibitors, as dual combination therapy, or with other OADs or insulin needs further evaluation. In our study, almost 11% of patients (n = 1118) were given the combination teneligliptin + SGLT2 inhibitor (such as empagliflozin, canagliflozin and dapagliflozin) together with other OADs. Kadowaki et al. [31] reported a significant improvement in the long-term safety and efficacy of canagliflozin as add‐on therapy to teneligliptin in Japanese patients, with the results suggesting the effectiveness of this combination in improving HbA1c, FPG and body weight.
We also analyzed the effectiveness of teneligliptin in patients with renal impairment (n = 549). Teneligliptin effectively improved the glycaemic parameters of these patients without worsening the eGFR after 12 weeks of treatment. In a study conducted by Haneda et al. [32], which was an interim report of the post-marketing surveillance over 2 years, long-term treatment with teneligliptin was found to be effective in reducing the glycaemic parameters and was also well tolerated in patients with any stage of renal impairments from normal to end-stage renal disease. We also analysed the effectiveness of teneligliptin in patients of different ages (three groups: < 60 years, 60–75 years and > 75 years) and found that teneligliptin effectively controlled glycaemic parameters in all age groups. Kadowaki et al. [33] demonstrated that there were no marked or clinically relevant differences in the reductions of FPG or HbA1c at 3 years across these three age subgroups (< 65, ≥ 65 to < 75, or ≥ 75 years ).
The biggest strength of this study is the large sample size (>10,000 Indian patients with T2DM), with clinically relevant data collected from a large number of Indian patients representative of the different regions of India. This makes it probably the largest study of real-world data on teneligliptin to date. One of the limitations of this study is its retrospective design, which does not allow determination of a cause-and-effect relationship due to the limited availability of the details of medical records. As this was a single-arm study that focussed on efficacy of teneligliptin, a safety assessment and comparative analysis were not possible.
Conclusion
Teneligliptin, a novel DPP-4I, when prescribed either as monotherapy or as an add-on to one or more commonly prescribed antidiabetic agents, significantly improved glycemic parameters. The results of this study suggest that teneligliptin can be considered to be an effective add-on antidiabetic agent in the management of Indian patients with T2DM.
References
Pandey SK, Sharma V. World diabetes day 2018: battling the emerging epidemic of diabetic retinopathy. Indian J Ophthalmol. 2018;66(11):1652–3.
International Diabetes Federation. IDF diabetes atlas, 9th edn. Brussels: International Diabetes Federation; 2019. https://diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf. Accessed 19 Mar 2020.
Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014;7(1):45–8.
Gupta V, Kalra S. Choosing a gliptin. Indian J Endocrinol Metab. 2011;15(4):298–308.
Chen XW, He ZX, Zhou ZW, et al. Clinical pharmacology of dipeptidyl peptidase 4 inhibitors indicated for the treatment of type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015;42(10):999–1024.
Kishimoto M. Teneligliptin: a DPP-4 inhibitor for the treatment of type diabetes. Diabetes Metab Syndr Obes. 2013;6:187–95.
Nabeno M, Akahoshi F, Kishida H, et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun. 2013;434(2):191–6.
Kadowaki T, Haneda M, Ito H, et al. Safety and efficacy of long-term treatment with teneligliptin: interim analysis of a post-marketing surveillance of more than 10,000 Japanese patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2018;19(2):83–91.
Pk KM, Ingole S, Tamboli T, Jain R. Management of type 2 diabetes mellitus: insights into prescribing trends. Int J Res Med Sci. 2017;5(4):1306–11.
Defronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–72.
Maladkar M, Sankar S, Kamat K. Teneligliptin: heralding change in type 2 diabetes. J Diabetes Mellit. 2016;6:113–31.
Ghosh S, Trivedi S, Sanyal D, Modi KD, Kharb S. Teneligliptin real-world efficacy assessment of type 2 diabetes mellitus patients in India (TREAT-INDIA study). Diabetes Metab Syndr Obes Targets Ther. 2016;9:347–53.
Nashikkar N, Panikar V, Joshi S, et al. Retrospective real-life efficacy assessment of teneligliptin in Indian T2DM patients. Int J Res Med Sci. 2018;6:3571–5.
Li X, Huang X, Bai C, et al. Efficacy and safety of teneligliptin in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2018;9:449.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–96.
Kutoh E. Differential effects of pioglitazone on metabolic parameters in newly diagnosed, drug-naive Japanese patients with type 2 diabetes with or without metabolic syndrome. Endocr Res. 2010;35(3):118–27.
Kutoha E, Hiratea M, Ikenoa Y. Teneligliptin as an initial therapy for newly diagnosed, drug naive subjects with type 2 diabetes. J Clin Med Res. 2014;6(4):287–94.
Raghavan V, Lahiri A, Akul SK, Goswami U, Gupta CN, Sen S. Effect of teneligliptin vs metformin on glycemic control in Indian patients with newly-diagnosed, drug-naïve type 2 diabetes mellitus: a 12-week randomized comparative clinical study. Int J Adv Med. 2019;6(2):481–8.
Agarwal P, Jindal C, Sapakal V. Efficacy and safety of teneligliptin in Indian patients with inadequately controlled Type 2 diabetes mellitus: a randomized, double-blind study. Indian J Endocrinol Metab. 2018 Jan;22(1):41.
Katsuno T, Ikeda H, Ida K, Miyagawa JI, Namba M. Add-on therapy with the DPP-4 inhibitor sitagliptin improves glycemic control in insulin-treated Japanese patients with type 2 diabetes mellitus. Endocr J. 2013;60:733–42.
Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013 Mar;15(3):252–7.
Lukashevich V, Schweizer A, Foley JE, Dickinson S, Groop PH, Kothny W. Efficacy of vildagliptin in combination with insulin in patients with type 2 diabetes and severe renal impairment. Vasc Health Risk Manage. 2013;9:21–8.
Tanaka S, Suzuki K, Aoki C, et al. Add-on treatment with teneligliptin ameliorates glucose fluctuations and improves glycemic control index in Japanese patients with type 2 diabetes on insulin therapy. Diabetes Technol Ther. 2014;16(12):840–5.
Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute fluctuations compared with sustained chronic hyperglucemia in patinets with type 2 diabetes. JAMA. 2006;295:1681–7.
Kadowaki T, Kondo K, Sasaki N, et al. Efficacy and safety of teneligliptin add-on to insulin monotherapy in Japanese patients with type 2 diabetes mellitus: a 16-week, randomized, double blind, placebo-controlled trial with an open-label period. Expert Opin Pharmacother. 2017;18(13):1291–300.
Narita T, Katsuura Y, Sato T, et al. Miglitol induces prolonged and enhanced glucagon-like peptide-1 and reduced gastric inhibitory polypeptide responses after ingestion of a mixed meal in Japanese type 2 diabetic patients. Diabet Med. 2009;26(2):187–8.
Arakawa M, Ebato C, Mita T, et al. Miglitol suppresses the postprandial increases in interleukin 6 and enhances active glucagon-like peptide 1 secretion in viscerally obese subjects. Metabolism. 2008;57:1299–306.
Lee A, Patrick P, Wishart J, Horowitz M, Morley JE. The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab. 2002 Sep;4(5):329–35.
Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diabetes Obes Metab. 2014 May;16(5):418–25.
Kadowaki T, Inagaki N, Kondo K, et al. Long-term safety and efficacy of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:77–84.
Haneda M, Kadowaki T, Ito H, et al. Safety and efficacy of teneligliptin in patients with type 2 diabetes mellitus and impaired renal function: interim report from post-marketing surveillance. Diabetes Ther. 2018 Jun;9(3):1083–97.
Kadowaki T, Haneda M, Ito H, Sasaki K, Yamada Y. Long-term safety and efficacy of teneligliptin in elderly patients with type 2 diabetes: subgroup analysis of a 3-year post-marketing surveillance in Japan. Adv Ther. 2020;37:2477–92.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were funded by Glenmark Pharmaceuticals Ltd, Mumbai, India.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Sujoy Ghosh developed the design of the study with valuable input from Mangesh Tiwaskar, Rajiv Chawla, Shalini Jaggi, Arthur Asirvatham and Vijay Panikar. The data were analysed and the manuscript prepared and reviewed by Sujoy Ghosh, Mangesh Tiwaskar, Rajiv Chawla, Shalini Jaggi, Arthur Asirvatham and Vijay Panikar.
Medical Writing, Editorial, and Other Assistance
We acknowledge the support of Digiscribe Technolgy Pvt Ltd for data management. We would also like to thank Rajeev Chawla, Arthur Ashirvadham, Faraz Farishta, G Satish Kumar, Ganga Kiran, H. Mohapatra, Ishaq Ahmed, Kirti Samudra, Mohammed Abubaker, Suhas Erande, Soumya Sengupta, Abhyudaya Verma, Rajeev Bansal, Ritesh Aggrawal, Sandeep Suri, Mangesh Tiwaskar, A Panneerselvam, Amit Rastogi for providing their valuable data for the analysis.
Prior Presentation
The abstract was presented as an E-poster at the International Diabetes Federation Congress 2019 (IDF 2019 Busan), 2–6 December, Busan, Korea.
Disclosures
Sujoy Ghosh, Mangesh Tiwaskar, Rajiv Chawla, Shalini Jaggi, Arthur Asirvatham and Vijay Panikar have nothing to disclose.
Compliance with Ethics Guidelines
Prior to collecting patient information approval was obtained from the Institutional Ethics Committee (Suraksha Ethics committee. Reg No: ECR/644/Inst/MH/2014/RR-17). The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12613196.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ghosh, S., Tiwaskar, M., Chawla, R. et al. Teneligliptin Real-World Effectiveness Assessment in Patients with Type 2 Diabetes Mellitus in India: A Retrospective Analysis (TREAT-INDIA 2). Diabetes Ther 11, 2257–2268 (2020). https://doi.org/10.1007/s13300-020-00880-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00880-4