Abstract
Introduction
Lipohypertrophies (LHs) due to incorrect insulin injection techniques have been described in the literature for decades. Their rate averages 38%, but this is still controversial because of the vast range reported by different publications, most of which fail to describe the selected detection protocol and therefore are not entirely reliable. We still need to identify the real LH rate, and only consistently using a standardized method in a large cohort of insulin-treated (IT) patients make this possible.
Methods
Our group performed thorough clinical skin examinations on patients suffering from type 2 diabetes mellitus (T2DM): 1247 IT T2DM outpatients were examined according to a standardized protocol, previously published elsewhere, as well as an ultrasound scan of the same skin areas to assess the degree of concordance between the two methods and to evaluate the demographic, clinical, and behavioral risk factors (RF) as well as metabolic consequences of identified LHs.
Results
The concordance between the two methods was 99%. Identified risk factors for LHs were needle reuse, failure to rotate injection sites, and ice-cold insulin injections. High HbA1c values, wide glycemic variability, and longstanding proneness to hypoglycemia with a high rate of ongoing hypoglycemic events proved to be significantly associated with LHs, too; the same applied to cardiovascular and renal complications as well as to living alone and being retired.
Conclusions
Based on a strict well-structured methodology, our data confirmed what has already been reported in the literature on factors leading to, or associated with, LHs and, for the first time in adults, indicated cryotrauma from ice-cold insulin injections and specific social conditions as factors facilitating LH occurrence. HCPs should therefore plan a yearly clinical examination of all injection sites to improve patient quality of life through better glucose control and a reduced rate of hypoglycemic events.
Trial Registration
Trial registration no. 127-11.01.2019, approved by the Scientific and Ethics Committee of Campania University “Luigi Vanvitelli,” Naples, Italy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lipohypertrophies (LHs) due to incorrect insulin injection techniques have been reported in the literature for decades at variable rates with an average of 38%. |
The extremely wide range of LH rates reported by different publications seems to depend mostly on poorly reproducible detection methods. |
Only the utilization of a standardized clinical method in a large cohort of insulin-treated patients can help provide a sufficiently reliable figure for the actual LH rate. |
After publishing a standardized LH identification protocol, we compared the results obtained using it with those of ultrasound (US) scans performed on the same skin areas in 1247 IT T2DM outpatients. |
The high degree (99%) of concordance between clinical and US results suggests routinely utilizing the former in diabetic outpatients to save much time and money. |
Our data confirmed what was already reported in the literature on several factors leading to or associated with LHs but, for the first time in adults, emphasized cryotrauma from ice-cold insulin injections and specific social conditions, such as retirement and living alone, as factors facilitating LH occurrence. |
Therefore, we suggest HCPs interested in this field should plan thorough yearly clinical examinations of all injection sites to improve patient quality of life through enhanced glucose control and a reduced rate of hypoglycemic events. |
Introduction
Skin lipodystrophy and lipohypertrophy (LH), its most prevalent (95%) form, are frequent localized diabetes treatment complications, known since the beginning of insulin therapy [1]. LHs are areas of thickened subcutaneous fat tissue confined to insulin injection sites in the form of painless indurations, swelling, and nodules lacking an external capsule and steadily growing over time with repeated injections. They tend to shrink after patients stop using the area for injections after being taught correct administration techniques.
LH histology has seldom been described so far. It consists of (1) macroadipocytes (the most represented cell population) and microadipocytes; (2) a tight weave of fibrin; (3) not more than the average number of inflammatory cells [2]. Recently, we published a case report on a large abdominal LH lesion with an insulin-rich fluid content [3]. Amyloid has seldom been detected in insulin injection sites, but no information is currently available on a possible correlation between the two [4].
From a recent meta-analysis reporting an average 38% prevalence [5], as well as from our data [6, 7], LH rates appear to differ greatly among different studies, ranging from 1.9% [8] to 73.4% [9]. While some authors are vague about their approach [10], others use LH diagnostic approaches that are different from one another . These include (1) patient- or nurse-completed questionnaires [11, 12] or (2) clinical skin examinations carried out by doctors, nurses, or other non-specialized healthcare professionals (HCP) whose identification methods and specific skills are not apparent from the text [5]. Some reports used ultrasonography (US) to assess LH diagnostic accuracy [13]. However, they dealt with rather small series and failed to define the LH morphology.
Highly competent investigators sometimes consider their LH identification methods as intuitively given in their publications, thus raising doubts about their own systematic approach to LH diagnosis and unwillingly contributing to the literature variability. Therefore, clinicians who have been poorly educated, if at all, about LHs during university studies are not prepared to correctly identify lesions and prevent patients from injecting insulin into them, thus further worsening glucose control [6]. This is why method reporting requires thoroughness.
These considerations led us to carry out a real-life investigation of the frequency of and risk factors for insulin-related LHs in a large cohort of patients with type 2 diabetes mellitus (T2DM), using a validated standardized clinical method. Another end point of our study was the identification of any relationships between US-based and clinical LH features. This study was conducted according to good clinical practice standards.
The study was carried out in accordance with the original Declaration of Helsinki and its later amendments and was approved by Vanvitelli University, Naples, Italy, and all the ethics committees of the centers participating in the study. (For the full list of participating centers, see the supplementary material.) Written informed consent was obtained from all participants before enrollment.
Methods
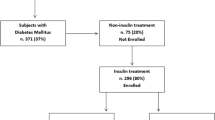
We conducted an open, multicenter, randomized, case-control study in a real-life setting by including all patients with T2DM referred consecutively to ten associated diabetes centers (DCs). The study did not include subjects with type 1 diabetes (T1DM) because: (1) the above-mentioned DCs were for adults and (2) we tried to prevent biases due to the two-to-four times longer insulin treatment extent than expected for T2DM.
T2DM diagnosis relied on criteria defined by the ADA Standards of Medical Care in Diabetes 2019 [14]. The International Classification of Diseases, Clinical Modification (ICD-9-CM, V82.9 2014), was used to classify comorbidities and complications related or unrelated to DM [15].
Eligibility criteria were: age > 18 years, at least 5-year duration of T2DM, and lifestyle intervention plus 3–4 (2–3 fast-acting and 1 basal) pen-based insulin analog injections per day for at least the last 12 months, without any added hypoglycemic agents.
Exclusion criteria were lack of autonomy or inability to fully understand and complete the questionnaire reported below. The Chronic Kidney Disease Epidemiology Collaboration (CKD-Epi) formula allowed us to record the estimated glomerular filtration rate (eGFR).
The questionnaire we used addressed socio-demographic and clinical characteristics including age, gender, school education, employment, living and marital status, possible caregiver role, pen needle features and re-utilization rate, injection site rotation habits, ice-cold insulin injection, post-injection leaking, local discomfort/pain/redness/itching or other skin abnormalities, and hypoglycemia during the past 12 months or symptomatic hypoglycemic episodes (HE) during the past 4 weeks, as described elsewhere [16]. All patients also entered a web-based clinical record form (eCRF), including clinical characteristics, habits, and demographics, as well as insulin injection habits. All subjects gave their written informed consent to participate in the study.
Based on the above criteria, out of the 3234 outpatients consecutively referred to the DCs, our study involved 1245 IT patients with T2DM. To avoid any possible biases due to missed LH areas, clinical (inspection and palpation) and US-based skin evaluations were performed at all possible sites (abdomen, arms, thighs, buttocks) as previously described [11], including those that individual patients said they had never used for injections. This way, after identifying 736 (59.5%) subjects with LHs, we excluded 18 for the inability to complete the questionnaire reliably. Finally, 718 patients with LH (from now on reported as LH +) entered the study along with 509 without LH (from now on reported as LH −).
Table 1 displays their overall features. Out of 64 patients with nephropathy, 21 had end-stage renal disease (ESRD) requiring dialysis and therefore entered an integrative follow-up program in dialysis units connected to DCs.
Hypoglycemia
We defined (1) severe hypoglycemia (SeH) according to the 2019 ADA guideline as an episode leading to unconsciousness or requiring assistance by a third person or associated with blood glucose levels < 54 mg/dl (3.0 mmol/l) or in the 56–70 mg/dl range [14]; (2) symptomatic hypoglycemia (SyH) as an episode characterized by at least one of the following symptoms resolving with food or sugary drink ingestion: palpitations, tremors, sweating, shakiness, irritability, concentration troubles, dizziness, hunger, blurred vision, confusion, tachycardia, or difficulty moving [13, 14, 16]; (3) frequent unexplained hypoglycemia (UH) as the occurrence of hypoglycemic episodes at weekly intervals or more, in the absence of any identified precipitating events, such as changes in insulin dosage, diet, or amount of physical activity [13, 14, 16].
Glycemic Variability
We defined glycemic variability (GV) as the mean blood glucose fluctuation occurring over the observation period in which patients controlled capillary pre- and 2-h-postprandial levels at each mealtime and midnight. They also had to check their capillary blood glucose (BG) level whenever feeling any symptoms suggestive of hypoglycemia.
All electro-medical devices used to evaluate BG were ISO-directive certified and periodically validated and verified by the DCs’ diabetes nurses [17]. In the absence of any user-friendly unanimously accepted clinical method, GV was investigated through a validated questionnaire [11, 13] and defined as high when BG levels swung consistently, inexplicably, and unpredictably from < 60 to > 250 mg/dl at least once a week over the 3 months preceding enrollment and at least 3 weeks within the first and second trimester of the study [13, 18].
LH Identification Training Protocol
Only trained HCPs with at least 3 years of specific experience performed the protocol, using a US jelly to enhance fingertip sensitivity as previously described and validated [7, 19]. It consisted of (1) the inspection of each area of interest using direct and tangential light against a dark background, taking into account the patient body position during injection, and (2) a thorough palpation technique involving slow circular and vertical fingertip movements followed by repeated horizontal attempts at the same spot. For abdominal examination, patients were lying down and stood up afterward; for thigh examination, they sat with bent legs and feet on the floor [5]. HCPs gently touched the skin at the beginning and then progressively increased the finger pressure. When perceiving harder skin, they performed a pinching maneuver to compare the thickness of suspected LHs to that of the surrounding areas [13] and repeated all maneuvers mentioned above in case of smaller and flatter lesions .
High-Frequency Skin Ultrasound Scans
High-frequency B-mode US skin scans were performed at all injection sites using the linear 20-MHz probe (Philips HD3) to define single LH features, including size, thickness, and texture, as described elsewhere [6, 7, 19], and to check palpation results as a secondary goal. Briefly, in our above-mentioned validation studies involving two different blinded operators on the same patients, a 100% consistency in LH identification was already apparent from the intra-operator, inter-operator, and day-to-day operator variation, independently of LH location, volume, extension, texture, or thickness.
According to US features, we classified LH areas as described elsewhere [20] into:
-
hyper-type A: iso-hyperechoic with a prevailing fibrotic component;
-
iso-type B: isoechoic associated with small edema-like islands bordered by fibrous strips;
-
iso-hypo type C: iso-hypoechoic fiber-free.
Also, although skin biopsy is the gold standard for this, US proved to be an easy-to-handle and accurate tool to rule out amyloid nodules, i.e., rare avascular, compact lesions with a hard-fibrous consistency [21].
Statistical Analysis
We reported patient characteristics as mean ± standard deviation (SD) for continuous variables or number/percentages for categorical variables.
SeH and SyH rates were included in the Poisson regression models and expressed as RRs within 95% confidence intervals (95% CI). We used the SAS Program (Release 9.4, SAS Institute, Cary, NC, USA) to examine variables associated with injection techniques by parametric and non-parametric tests (i.e., repeated measures analysis of variance integrated by a two-tailed paired Student’s t test with 95% CI and Mann-Whitney U test, respectively) as needed and to analyze associations among categorical variables by the χ2 test with Yates correction or Fisher's exact test. We accepted p < 0.05 as the least level of statistical significance.
Results
In our study, LHs were not present in any areas other than those used by patients to inject insulin. We describe participants’ clinical characteristics, injection habits, and demographics in Table 1, which clearly shows some differences between the two groups. LH + subjects had significantly greater HA1c values than LH – (9.6 + 1.1% vs. 7.5 + 1.1, respectively; p < 0.001) as well as higher daily insulin dose requirements (54.9 ± 8.2 vs. 42.3 ± 10.0; p < 0.01), larger glycemic variability (287 ± 76 vs. 198 ± 54 mg/dl; p < 0.01), more frequent HEs and UH in terms of percent of subjects involved (97.6% vs. 66.6%, p < 0.001 and 82.9% vs. 21.4%; p < 0.0001, respectively), cardio-/cerebrovascular complications (28.5% vs. 17.5%, p < 0.01), or nephropathy including dialysis treatment (38.5% vs. 17.9%, p < 0.01). As for injection technique habits, LH + subjects consistently injected insulin into LH nodules (100%) and reused needles more than LH – subjects (97.8% vs. 18.7%; p < 0.0001). They also failed to rotate injection sites (97.5% vs. 30.4%, p < 0.001), self-injected ice-cold insulin (72.3% vs. 32.1%, p < 0.01), noticed post-injection drop leaking from the needle (43.2% vs. 29.5%, p < 0.01), and did not experienced any pain when injecting (0.7% vs 26.2%, p < 0.01) more often than the LH – patients. The LH + group was less likely than the LH – group to be employed (20.7% vs. 38.4%; p < 0.01), mostly because of retirement (60.1 vs. 42.3%; p < 0.01), and lived alone more often (18.8% vs. 10.9%, p < 0.05). Needle length and time spent before removing the needle from the skin at the end of the injection did not differ between groups.
We report the amount, location, and texture of LHs according to clinical features in Table 2, as already described elsewhere [19]. Skin lesions identified by clinical skin examinations and US were almost concordant (99% cases). LH distribution was approximately the same in the abdomen (38.3%), arms (35.8%), and thighs (33.3%), but significantly lower on the buttocks (26.2%, p < 0.05); 45% of patients had LHs at only one site, 35% at two sites, and 20% at more than two sites; 57% of patients had bilateral symmetrically arranged LHs. Abdominal LHs were predominantly protruding and ≥ 4 cm; limb LHs were mostly protruding and < 4 cm, while those found on the buttocks were flat and < 4 cm.
Based on inspection/palpation features, we arbitrarily identified four LH types, as illustrated in Fig. 1:
-
A type = easily seen and palpable protruding nodules < 4 cm with a harsh elastic texture;
-
B type = easily seen and palpable protruding nodules ≥ 4 cm with a harsh-elastic texture;
-
C type = slightly protruding flat nodules with an elastic texture and mostly requiring pinching because of their hardly seen and palpable structure;
-
D type = have a hard-elastic texture, quite tricky to identify because they are difficult to see and only detectable through deep palpation and pinching.
As differences in site, size, morphology, and texture have led to the vast LH rate underestimation observed in the literature [7, 19], we also tried to facilitate future lesion identification by more precisely defining LH structure through a careful head-to-head comparison between clinical and US-based features. Table 3 describes the results of this analysis:
-
Clinically defined type A LHs pertained mostly to the hyper-type A US class (by 64%);
-
Clinically defined type B LHs pertained mostly to the hyper-type A US class (by 57%);
-
Clinically defined type C LHs pertained to the iso-type B class in 48% and to the iso-hypo-type C class in 41%;
-
Clinically defined type D LHs pertained to the hyper-type A class in 51%.
Table 4 summarizes only parameters significantly associated with LH risk (RR, 95% CI) grouped under different categories, including elements contributing to the overall injection technique.
Within the first parameter category, the following elements attained statistical significance: HbA1c (95% CI 1.88–3.87, RR 2.96), SeH episodes occurring during the past 12 months (95% CI 1.56–3.63, RR 2.62), cardiovascular complications (95% CI 2.18–4.49, RR 3.36), and end-stage renal disease (ESRD)/dialysis (95% CI 1.59–3.23, RR 2.69).
A highly significant association was also found with HEs, both as a whole (95% CI 1.97–3.83, RR 2.96) and separately as SeH (95% CI 2.18–4.14, RR 3.59) or SyS (95% CI 1.98–3.16, RR 2.71) as well as with GV (95% CI 2.12–4.38, RR 3.16).
For major injection technique defects, those still significantly associating with the risk of LH were: needle reuse (95% CI 3.18–5.78, RR 4.43), failure to rotate injection sites (95% CI 3.12–6.28, RR 5.11), ice-cold insulin injection (95% CI 2.12–4.81, RR 3.06), and post-injection drops leaking from the needle (95% CI 1.99–2.45, RR 1.68). Failure to keep the needle in the skin for 20 s after the injection did not have an association with LH risk (95% CI 0.87–1.58, RR 1.09).
Also living alone (95% CI 1.10–1.57, RR 1.36) and acting as caregivers (95% CI 1.10 – 1.62, RR 1.44) attained statistical significance. Within the employment status category, only being retired had some relevance (95% CI 1.07–1.93, RR 1.52).
Finally, Table 5 describes HEs, divided into SeH and SyH. SeH represented about 16% of the total. All parameters under study were included in a stepwise multiple regression analysis to identify hypoglycemia-associated factors. As shown, SeH was strictly associated with a LH diameter ≥ 4 cm (RR 3.36; 95% CI 2.33–5.28), especially in the case of abdominal or multiple sites (RR 2.24, 95% CI 1.45–2.68; RR 2.35, 95% CI 1.97–2.89, respectively). We also detected a strong association of SeH with intranodular injections (RR 3.86; 95% CI 2.48–4.25), needle reuse (RR 3.18, 95% CI 2.12–3.79), missed injection site rotation (RR 3.52, 95% CI 2.89–4.66), > 40 IU daily insulin requirement (RR 1.75, 95% CI 1.16–2.08), > 6-mm-long needles (IR 2.06, 95% CI 0.88–2.98), and ice-cold insulin utilization (RR 1.85, 95% CI 1.25–2.86). SyH was also strongly associated with the same factors except for LH size and needle length but with the addition of post-injection drop leaking (RR 1.32, 95% CI 0.98–1.98).
Discussion
A recent meta-analysis on 12,493 subjects from 26 RCTs describes an average LH prevalence rate as high as 38% among insulin-treated subjects with large between-study heterogeneity [5]. This indicates a widespread educational problem concerning LH prevention among patients. As suggested by the differeing and incomplete descriptions of LH identification methods across the literature, with just a few exceptions [7, 19, 22], the reason for the lack of education might be clinicians feeling more comfortable with drug prescription than with administration advice [13] despite the broad dissemination of updated guidelines in the field [21, 23].
Most authors agree that LHs are invariably associated with the following factors: failure to rotate injection sites, needle reuse, female gender, high body mass index (BMI), and long disease duration. A higher daily insulin dose requirement is still a matter of debate, however. Our results, while confirming those associations, added ice-cold insulin injection to the list, suggesting that repetitive thermic trauma amplifies the damages caused by mechanical ones. Cryotrauma caused by ice-cold insulin injection has already been described in the literature as a risk factor (RF) for lipodystrophies in children and adolescents [24], with particular reference to lipoatrophy rather than LH, while for the first time our data point to an association between cryotrauma and LHs in adults.
The association of LHs with metabolic parameters including high HbA1c levels and large GV [5, 6, 8, 9, 12, 13, 18, 22, 25] along with increased risk of severe and non-severe hypoglycemia have already been reported in the literature, which also applies to other factors that our results confirmed together with chronic diabetes complications, especially renal and cardiovascular ones. The latter finding demands special attention to an eventually additive LH-related health hazard, as frequent HE and extensive GV are also known to be independent cardiovascular complication factors [26,27,28,29].
Another piece of information we obtained from our data was the association of LHs not only with a long-standing history of HE but also with living alone and retired status; specific investigations are needed to confirm and interpret these findings, which suggest a possible role of a creeping depression, which is frequently associated with these conditions [30].
Despite the many current concerns and unmet needs regarding this topic, we firmly believe that, with patients adequately positioned, careful direct or tangential light-assisted inspection of all injection sites [5, 6] along with repeated superficial and deep jelly-enhanced palpation is crucial to identify LHs, if necessary followed by pinching for less elastic skin areas [13].
We also found that the abdomen was most often chosen for insulin injections, followed by the arms, thighs, and buttocks [13, 31]. Further studies are warranted to interpret this observation, considering that time is crucial for LH generation, and, according to our long-standing personal unpublished observation, lesions become increasingly more frequent and extensive with long-lasting repeated insulin injections into the same site. We already published a paper with several didactic pictures of LH explaining why, and how, to best use the positioning and light angle for diagnostic purposes [6]. Based on our experience, we arbitrarily grouped LHs into four primary identification feature-based categories, as reported in Fig. 1, which proved useful for both HCP and patient training.
Comparisons between LH palpation and US results have been inconsistent so far: one group reported 65% more false-positive results with palpation [32], and another found just the opposite, detecting 50% more LHs with US [33]. It is conceivable that palpation-based LH detection problems depend on inadequate training, which our daily practice clearly shows impairs the ability to identify LH. In fact, despite trying to comply with published guidelines, most notably the 2010 FIT guideline [34], many groups have obtained weaker results so far than would be expected if they had followed the standardized protocol we published over the last few years [7, 19].
Recently, a group using a strict methodology found US to have higher accuracy than palpation [35], thus suggesting it as the gold standard for LH diagnosis [5, 19]. Undoubtedly, US has additional advantages over palpation by allowing clinicians to provide patients with better advice based on the LH details, including the size, distribution, elastic/fibrous texture, and possible fluid content [35,36,37,38]. However, as already expected from our preliminary reports [19, 36], our clinical method was virtually as accurate as US. Therefore, when systematically adopted, it may be very efficient and even preferable, considering its ability to reduce the use of US in daily clinical practice as US, when extended to the vast outpatient setting, is expensive, time-consuming, and therefore inappropriate.
Currently, studies comparing clinical detection with US accuracy are limited. We thought it useful to compare four clinically defined variants with three US-based classes in an attempt to predict fibrous/edematous LH structures causing insulin pharmacokinetic and pharmacodynamic changes related to intra-nodular injections [39, 40]. When injected into nodules resembling fiber-encapsulated reservoirs, no pain occurs, as if these skin areas were denervated and insulin could enter the blood to a variable extent according to either (1) delayed or (2) sudden and unpredictable release patterns, with extensive GV or sudden hypoglycemia as expected consequences, respectively. Consequently, as already published by our group, when switching to healthy skin, patients should reduce daily doses by at least 15–20% [13, 31] while intensifying self-monitoring of blood glucose levels for a while to optimize treatment [13].
Only anecdotal data from two clinical cases we recently reported support this view. The first described the case of a woman experiencing several SeHs, sometimes even having seizures, who had two large symmetrical abdominal LHs systematically used for insulin shots [3]. US scans revealed fluid content within the nodules containing a 13-fold insulin concentration compared to the blood. An intensive training on correct LH detection and insulin injection techniques prevented her from injecting insulin into LHs any longer and stopped the episodes of SeH, while even reducing her daily doses by > 25%. The second case was another woman with an abdominal LH referred to our unit for frequent unexplained HE [41, 42]. Wanting to wear a bikini when going to the beach, one morning she tried to get rid of her ugly nodule by massaging it with soothing emollient ointments, then she suddenly woke up in the hospital emergency room after a SHE; her husband confirmed that the above-mentioned LH had been the site of her last insulin injection. A careful education session on correct LH handling helped her avoid further HEs.
Limitations
The interpretation of LH pathophysiology in terms of fibrosis and edema is still hypothetical and based only on anecdotal reports. Therefore, further studies are needed to further support this view. Nevertheless, LH structural features can easily support the explanation of otherwise unexpected HEs.
LH classification into four clinically relevant groups is arbitrary. Still, it helps both to explain why inspection alone is mostly ineffective and to teach patients what LHs are and how to perform self-examination maneuvers to detect them.
Another limitation of the study comes from the exclusion of subjects with T1DM to rule out methodologic biases related to the two-to-four times longer insulin treatment extent than expected for people with T2DM. Also, as our patients only used insulin analogs, we could not compare LH rates associated with them to those associated with human regular, NPH, or premixed insulin preparations, which often were used instead, in studies published by other investigators. However, many studies found no significant differences among the types of insulin concerning the association with LHs [43,44,45,46,47,48].
Conclusion
In conclusion, from our findings we developed the following ten golden rules:
-
(1)
LHs have different morphologies and structures and in our series were only found at injection sites;
-
(2)
they are not always recognizable at first sight, so an accurate palpation and pinching maneuver is essential for detection;
-
(3)
direct and tangential light orientation with respect to the skin can make LH identification easier [6];
-
(4)
positioning (i.e., standing, sitting, or lying) is also essential to identify LHs [6];
-
(5)
using a lubricant can enhance fingertip sensitivity;
-
(6)
careful inspection/palpation of injection sites can allow trained health care professionals to achieve a similar degree of accuracy as that observed with skin US scans;
-
(7)
specific theoretical and hands-on LH identification training under the guidance of fully experienced personnel is essential to achieve the needed diagnostic accuracy;
-
(8)
factors associated with LHs were: (1) failure to rotate injection sites, needle reuse, long disease duration, higher HbA1c levels, more severe and more frequent micro- and macrovascular complications (thus supporting findings from other groups), and (2) history of SEH, ice-cold insulin injection, and having single and retired status (identified for the first time);
-
(9)
LH consequences were confirmed: more frequent SeH or SyH and high GV;
-
(10)
although US can be seen as the gold standard for LHs detection, its routine utilization is too expensive and time consuming to be proposed for an outpatient setting, but might be very useful for both health personnel and patients to identify the structure of a specific nodule.
Also, our results show that living alone and being retired were associated with LHs. A likely explanation is that these two social/psychologic conditions, which notoriously lead to depression and poor self-care, adversely affect insulin injection behavior. This hypothesis deserves further support from detailed psychologic investigations, but still suggests the clinician should be cautious and take this aspect into proper account when prescribing insulin.
The most crucial point is that it is unacceptable nowadays for insulin-treated patients not to know how to handle an insulin pen and which kind of needle is recommended by guidelines or how and where to inject insulin. Therefore, it is essential to make every effort to improve patient education on this topic to improve diabetes control and quality of life.
Our final suggestion to all HCPs is to plan a thorough clinical examination of all injection sites at least annually and involve patients in this task after educating them about both the best LH identification methods and the reasons for carefully avoiding injections in nodules. This strategy has proven effective in our hands, as this way patients become strongly motivated to collaborate with HCPs after verifying a 20% decrease in the daily insulin dose requirement with lower HbA1c levels and especially after experiencing better quality of life due to fewer HEs and a less prominent GV.
References
Depisch F. Über lokale lipodystrophie bei lange zeit mit insulin behandelten fällen von diabetes. Klin Wochenschr. 1926;42:1965–6.
Fujikura J, Fujimoto J, Yasue S, et al. Insulin-induced lipohypertrophy: report of a case with histopathology. Endocrine J. 2005;52(2):623–8.
Gentile S, Strollo F, Della Corte T, Marino G, Guarino G, Italian Study Group on Injection Techniques. Skin complications of insulin injections: a case presentation and a possible explanation of hypoglycaemia. Diabetes Res Clin Pract. 2018;138:284–7. https://doi.org/10.1016/j.diabres.2018.02.005.
Ansari AM, Osmani L, Matsangos AE, Li QK. Current insight in the localized insulin-derived amyloidosis (LIDA): clinico-pathological characteristics and differential diagnosis. Pathol Res Pract. 2017;213(10):1237–41. https://doi.org/10.1016/j.prp.2017.08.013.
Gentile S, Strollo F, Guarino G. Why are so huge differences reported in the occurrence rate of skin lipohypertrophy? Does it depend on method defects or on lack of interest? Diabetes Metab Syndr. 2019;13(1):682–6. https://doi.org/10.1016/j.dsx.2018.11.042.
Deng N, Zhang X, Zhao F, Wang Y, He H. Prevalence of lipohypertrophy in insulin-treated diabetes patients. A systematic review and meta-analysis. J Diabetes Investig. 2017;9:536–43.
Gentile S, Strollo F, Guarino G, On behalf of the AMD-OSDI Italian Injection Technique Study Group, et al. Factors hindering correct identification of unapparent lipohypertrophy. J Diabetes Metab Disord Control. 2016;3(2):42–7. https://doi.org/10.15406/jdmdc.2016.03.00065.
Pavlovic MD, Milenkovic T, Dinic M, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30:1964–7.
Li FF, Shi-Min F, et al. Injection sites lipohypertrophy among 736 patients with type 2 diabetes of different-grade hospitals. Int J Clin Exp Med. 2016;9:13178–83.
Vardar B, Kizilci S. Incidence of lipohypertrophy in diabetic patients and a study of influencing factors. Diabetes Res Clin Pract. 2007;77:231–6.
De Coninck C, Frid A, Gaspar R, et al. Results and analysis of the 2008–2009 insulin injection technique questionnaire survey. J Diabetes. 2010;2:168–79.
Berard L, Cameron B. Injection technique practices in a population of Canadians with diabetes: results from a recent patient/diabetes educator survey. Can J Diabetes. 2015;39:146–51.
Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39:445–53.
ADA Classification and diagnosis of diabetes. Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. https://doi.org/10.2337/dc19-Sint01.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). National Center for Health Statistics. https://www.cdc.gov/nchs/icd/icd9cm.htm Accessed Jan 2018.
Giorda CB, Ozzello A, Gentile S, et al. Incidence and correlates of hypoglycemia in type 2 diabetes. The Hypos-1 Study. J Diabetes Metab. 2014;5:344. https://doi.org/10.4172/2155-6156.1000344.
Pleus S, Baumstark A, Jendrike N, et al. System accuracy evaluation of 18 CE-marked current-generation blood glucose monitoring systems based on EN ISO 15197:2015. BMJ Open Diabetes Res Care. 2020;8(1):e001067. https://doi.org/10.1136/bmjdrc-2019-001067.
Strauss K, De Gols HD, Hannet I, et al. A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Pract Diabetes Int. 2002;19:71–6. https://doi.org/10.1002/pdi.314.
Gentile S, Guarino G, Giancaterini A, Guida P, Strollo F, AMD-OSDI Italian Injection Technique Study Group. A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin-treated people with diabetes. Springerplus. 2016;5:563. https://doi.org/10.1186/s40064-016-1978-y.
Bertuzzi F, Meneghini E, Bruschi E, Luzi L, Nichelatti M, Epis O. Ultrasound characterization of insulin induced lipohypertrophy in type 1 diabetes mellitus. J Endocrinol Invest. 2017;40(10):1107–13. https://doi.org/10.1007/s40618-017-0675-1Epub 2017 Apr 27.
Gentile S, Grassi G, Armentano V, et al. AMD-OSDI Consensus on Injection Techniques for People with Diabetes Mellitus. Med Clin Rev. 2016, 2:3. https://medical-clinical-reviews.imedpub.com/amdosdi-consensus-on-injection-techniques-for-people-with-diabetes-mellitus.pdf. Acessed 30 Apr 2020.
Ji L, Sun Z, Li Q, et al. Lipohypertrophy in China: prevalence, risk factors, insulin consumption, and clinical impact. Diabetes Technol Ther. 2017;19(1):61–7. https://doi.org/10.1089/dia.2016.0334.
Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231–55. https://doi.org/10.1016/j.mayocp.2016.06.010.
Mahmud FH, Elbarbary NS, Fröhlich-Reiterer E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Other complications and associated conditions in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2018;19 Suppl 27(Suppl 27):275–286. doi:10.1111/pedi.12740
Cunningham MT, Mckenna M. Lipohypertrophy in insulin-treated diabetes: Prevalence and associated risk factors. J Diabetes Nur. 2013;17:340–3.
Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diab Technol Ther. 2012;14(Suppl 1):S51–S5858. https://doi.org/10.1089/dia.2012.0031.
Zhou JJ, Schwenke DC, Bahn G, Reaven P, VADT Investigators. Glycemic variation and cardiovascular risk in the veterans affairs diabetes trial. Diabetes Care. 2018;41(10):2187–94. https://doi.org/10.2337/dc18-0548.
Liang S, Yin H, Wei C, Xie L, He H, Liu X. Glucose variability for cardiovascular risk factors in type 2 diabetes: a meta-analysis. J Diabetes Metab Disord. 2017;16:45. https://doi.org/10.1186/s40200-017-0323-5.
Hanefeld M, Frier BM, Pistrosch F. Hypoglycemia and cardiovascular risk: is there a major link? Diabetes Care. 2016;39(Suppl 2):S205–S209209. https://doi.org/10.2337/dcS15-3014.
Schmitt A, Reimer A, Kulzer B, Haak T, Gahr A, Hermanns N. Negative association between depression and diabetes control only when accompanied by diabetes-specific distress. J Behav Med. 2015;38(3):556–64. https://doi.org/10.1007/s10865-014-9604-3.
Hirsch LJ, Strauss KW. The injection technique factor: what you don't know or teach can make a difference. Clin Diabetes. 2019;37(3):227–33. https://doi.org/10.2337/cd18-0076.
Partenen TM, Rissanen A. Insulin injection practice. Pract Diabetes. 2000;17(8):252–4.
Volkova N. Ultrasonography of insulin injection sites in diabetic patients: a new method of lipohypertrophy diagnostics. Endocrine reviews. Conference: 95th annual meeting and expo of the Endocrine Society, 2013; 34(3 Suppl.1).
Forum for Injection Technique UK (FIT UK). The UK injection and infusion technique recommendations, 4th edn, 2016. http://www.fit4diabetes.com/files/4514/7946/3482/FIT_UK_Recommendations:_4th_Edition.pdf. Accessed 12 Apr 2020.
Abu Ghazaleh H, Hashem R, Forbes A, et al. A systematic review of ultrasound-detected lipohypertrophy in insulin-exposed people with diabetes. Diabetes Ther. 2018;9(5):1741–56. https://doi.org/10.1007/s13300-018-0472-7.
Perciun R, Mihu M. The subcutis ultrasound map of type 1 diabetic children improves the diagnosis of local dystrophies and insulin injection technique. Pediatr Res Int J. 2014;2014(10):402780.
Kapeluto J, Paty BW, Chang SD, Eddy C, McNeilly G. Criteria for the detection of insulin-induced lipohypertrophy using ultrasonography. Can J Diabetes. 2015;39(6):534.
Kasperska-Czyzyk T, Stefanski P, Elwertowski M. Ultrasonographic assessment of subcutaneous lipohypertrophy at insulin injection sites. Diabetes Res Clin Pract. 2000;50:78.
Gentile S, Agrusta M, Guarino G, et al. Metabolic consequence of incorrect insulin administration techniques in aging subjects with diabetes. Acta Diabetol. 2011;48:121–5. https://doi.org/10.1007/s00592-009-0172-x.
Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 1486e;39:1486e92.
Improta MR, Strollo F, Gentile S. Lessons learned from an unusual case of severe hypoglycemia. Diabetes Metab Syndr. 2019;13(2):1237–9. https://doi.org/10.1016/j.dsx.2019.01.033.
Baruah MP, Kalra S, Bose S, Deka J. An audit of insulin usage and insulin injection practices in a large Indian cohort. Indian J Endocrinol Metabol. 2017;21(3):443.
Ajlouni MA, Abujbara M, Batieha A, Ajlouni L. Prevalence of lipohypertrophy and associated risk factors in insulin-treated patients with type 2 diabetes mellitus. Int J Endocrinol Metab. 2015 Apr;13(2):e20776.
Al Hayek AA, Robert AA, Braham RB, et al. Frequency of lipohypertrophy and associated risk factors in young patients with type 1 diabetes: a cross-sectional study. Diabetes Ther. 2016;7:259–67. https://doi.org/10.1007/s13300-016-0161-3.
Buyruk BA, Kebapci N, Yorulmaz G, Alaguney ES, Akalin A, Efe B. Prevalence and risk factors of lipohypertrophy and lipoatrophy in diabetes patients receiving insulin therapy. Diabetes. 2019. https://doi.org/10.2337/db19-59-LB.
AlJaber AN, Sales I, Almigbal TH, Wajid S, Batais MA. The prevalence of lipohypertrophy and its associated factors among Saudi patients with type 2 diabetes mellitus. J Taibah Univ Med. 2020. https://doi.org/10.1016/j.jtumed.2020.03.006.
Barola A, Tiwari P, Bhansali A, Grover A, Dayal D. Insulin-related lipohypertrophy: lipogenic action or tissue trauma? Front Endocrinol. 2018. https://doi.org/10.3389/fendo.2018.00638.
Forum for injection Technique (FIT). https://www.fit4diabetes.com/about-this-site/. Accessed 30 April 2020.
Acknowledgements
Special thanks are due to Dr. Paola Murano of Nefrocenter Research for the continuous logistical support and to members of AMD-OSDI Study Group on Injection Technique for editorial assistance and critical manuscript revision. Our sincerest thanks go to the doctors, nurses, and patients of the participating centers.
Members of the Nefrocenter Research and Nyx Start-up Study Group
Nephrologists: S. Meccariello, F. Crisci, F. Leone, R. Marino, M. Romano, G. Cristiano, E. Riccio, F. D'Anna, F. Borghesi, L. Di Gennaro, I. Raiola, A.M.La Manna, M. Cicala, P. Boccia, G. Garofalo, T. Castellano, V. Fimiani, A. Girone, A. Angelino, R. Di Livio, S. Celentano, L. Petruzzelli, L. Annichiarico, A. Cuomo, G. Latte, S. Oliviero, I. Capuano, L. Scarpati, G. Lubrano, L. Giordano, R. Sorrentino, M. Di Monte, F.A. Savino, M.L. Abategiovanni, P. Vendemia, A. Caiazza, A. Scarfato, A. Visone, D. Porpora, R. Mazzarella, C. Botta, O. Di Gruttola, L. Sorrentino, S. Kseniya, S. Vitale I. Cupic, V. Di Stazio, E. Satta, G. Monte, G. Barbuto, F. D'Errico, A. Ciccarelli, E. Verrillo, M. Nappo, A. De Maio, P. Miano, E. Trapanese, G. Brengola, R. Reggio, T. Pagano, N. Crispino, P. Napolitano, R.Cipriano, F. Secondino, Mercogliano, F. Salemi, C. Alfarone, G. Romano, L. Di Leva, R. Barretta. Diabetologists: S. Gentile, G. Guarino, F. Strollo, A. Vetrano, C. Martino, A. Fasolino, MR. Improta, G. Cozzolino, M. Corigliano, C. Brancario, A. Sellere, C. Lamberti, A, Vecchiato. Nurses: V. Morgillo, V. Morgillo, M. Fusco, A. Cimmarosa, M. Brida, A. Guerra, P. Spallieri, P. Cioffi, G. Massaro, C. Romano, R. Apuzzo, A. Cherillo, G. Erbaggio, G. Indaco, L. Manzo, E. Menna, A. Izzo, A. Di Matola, F. Fontanella, M. Puce, G. Fierro, E. Russo, A. Pascarella, A. Di Nardo, A. Bartiromo, T.J. Pazdior, A. Palmiero, E. Ruotolo, R.V. Amoroso, O. Belardo, P. Como, M.T. Natale, L. Erpete, A. Occhio, P.P. Tignola, M. Capasso, F. Barbaro, L. Erpete, S. Milano, M. Strazzulli, A. D'Errico, M.E. Toscano, C. Cirillo, C. Tabacco, A. Stasio, D. Palmeri, M.A. De Vita, A. Auletta, G. Cozzolino, M. Migliaccio, A. Iannone, I. Silvestri, V. Bianco, G. Barrella, T. Conturso, T. Cesarini, O, Ferraro, M. Festinese, L. Bellocchio, V. Pettinati, G. Felaco, E. Ebraico, M. De Lucia, A.M. Mandato, G. Di Maio, M. Cicchella, E. Cicchella, G. Casoria, A. Ricuperati, G. Calabrese, F.M. Isola, M.A. Cesarano, M. Di Riso, M.J. Mlynarska, L. Ambrosino, H. Buska, G. Esposito, V. Esposito, A. Pandolfo, V. D'Esculapio, A. De Costanzo, S. Caso, I. Kropacheva, A. Casaburo, A. Pellino, A. Rainone, C. Gigante, L. Imbembo, T. Carrara, P. Alibertini, L. Bottiglieri, C. D'Elia, C. Montesarchio, B. Jeschke, Z, Matusza, L. Orropesa, M. Vitale, M. Roselli, G. Buonocore, M. Siani, C. Giove, E. Petrone, F. Russo, A. Salsano, M. Agrisani, D. Giordano, A. Crispino, S. De Felice, G. Garofalo, D. Doriano, E. Di Virgilio, G. Fiorenza, I. Mattiello, L. Gala, R. Erricchiello, L. De Micco, I. Fioretti, R. Gladka, D. Mannato, T. Esposito, A. Schettino, R. Riccio, A. Allocca, G. Rusciano, C. Imbimbo, G. Ummarino, G. Fiorenza, G. Salzano, E. De Vincentis, I. Mattiello, P. Ferrante, V. Passa, A. Siani, A. Pastore, M. Battipaglia, G. Martone, E. Della Monica, G. Bernardinelli, D. Battipaglia. Nutritionist: T. Della Corte.
Memebrs of the AMD-OSDI Sudy Group
De Riu S, De Rosa N, Grassi G, Garrapa G, Tonutti L, Speese K, Cucco Cucco L, Branca MT, Botta A.
Funding
The paper was supported by a non-conditioning special grant from Nefrocenter Research Network and NYX Startup, Naples, Italy. None of the authors or co-workers received funding or another type of payment for this paper.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
SG and FS created the paper and wrote it. ES, TDC, ES, CR, CA, GG, GM, AF, GC, SC, MP, MC, EM, DO, RS, CR, and SV critically revised and approved the paper. All collaborators complied with data collection, critically assessed the results, and approved the final text.
Disclosures
Sandro Gentile, Giuseppina Guarino, Teresa Della Corte, Giampiero Marino, Alessandra Fusco, Gerardo Corigliano, Sara Colarusso, Marco Piscopo, Marco Corigliano, Emilia Martedi, Domenica Oliva, Viviana Russo, Rosa Simonetti, Ersilia Satta, Carmine Romano, Carmelo Alfarone, Antonio Vetrano, Carmine Martino, Clelia Lamberti, Agostino Vecchiato, Giuseppe Cozzolino, Clementina Brancario, and Felice Strollo have no financial interests to declare in relation to the present study.
Compliance with Ethics Guidelines
This study was conducted in conformance with good clinical practice standards. The study was led in accordance with the original Declaration of Helsinki and its later amendments and was approved by Vanvitelli University, Naples, Italy, and all the ethics committees of the centers participating in the study. (For a full list of participating centers, see the supplementary material.) Written informed consent was obtained from all participants before enrollment.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12555584.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gentile, S., Guarino, G., Corte, T.D. et al. Insulin-Induced Skin Lipohypertrophy in Type 2 Diabetes: a Multicenter Regional Survey in Southern Italy. Diabetes Ther 11, 2001–2017 (2020). https://doi.org/10.1007/s13300-020-00876-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00876-0