Abstract
Introduction
This study was designed to investigate the effectiveness of a video-based lifestyle education program (VBLEP) in improving glycemic control in people with type 2 diabetes mellitus compared with usual care.
Methods
Patients on stable oral glucose-lowering agents for at least 3 months and HbA1c 7.5–10% were randomized in a 1:1 ratio. Primary outcome measure was the difference in change in mean HbA1c between groups.
Results
The participants (n = 81) had mean (± SD) age of 50.1 (± 9.4) years and HbA1c of 8.5 ± 0.7% (68.87 ± 7.56 mmol/mol). The follow-up data were available in 96% (78/81) of participants. Of 40 participants, 36 (90%) attended ≥ 75% (≥ 3 out of 4) of the sessions in the VBLEP. In the intention-to-treat analysis, a significant reduction [0.6% 95% CI (0.1, 1.1), p = 0.013] in HbA1c was seen in the VBLEP group compared with usual care. A ≥ 1% reduction in HbA1c was observed in 39.5% of participants in the VBLEP compared with 15% in the usual care arm. However, a ≥ 0.5% reduction in HbA1c was observed in 65.8% of participants in the VBLEP compared with 37.5% in the usual care arm (p = 0.012). There was a significant change in weight and body mass index in the VBLEP group compared with usual care. The participants who were employed, had a family history of diabetes, had no diabetes-related complications, and were in the VBLEP group had higher odds of having a favorable HbA1c reduction (≥ 0.5%, combined analysis both groups) from baseline.
Conclusion
The VBLEP demonstrated a significant and clinically relevant HbA1c reduction compared with usual care. A simple VBLEP, when delivered in an interactive manner, can aid in improving glycemic outcomes in the Indian population.
Trial Registration
CTRI/2017/05/008564.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There have been no data on a video-based lifestyle education program (VBLEP) from India for management of diabetes. |
This study was carried out to determine the effectiveness of a video-based lifestyle education program delivered to participants with uncontrolled diabetes. |
What was learned from the study? |
This study found that on intention-to-treat analysis, a significant reduction [0.6% 95% CI (0.1, 1.1), p = 0.013] in HbA1c was seen in the VBLEP group. |
A ≥ 1% reduction in HbA1c was observed in 39.5% of participants in the VBLEP compared with 15% in the usual care arm. A ≥ 0.5% reduction in HbA1c was observed in 65.8% participants in the VBLEP compared with 37.5% in the usual care arm. |
Presence of diabetes-related complications was less likely to be associated with achievement of a favorable outcome of ≥ 0.5% reduction in HbA1c. |
Introduction
Type 2 diabetes mellitus (T2DM) has become a major health burden globally. Medical nutrition therapy (MNT) and physical exercise are essential pillars for the management of diabetes [1]. However, adherence to these lifestyle measures has been sub-optimal in usual care for diabetes [2], resulting in poor glycemic control and a higher rate of diabetes-related morbidity and mortality [3]. To improve outcomes for patients with diabetes, structured lifestyle intervention programs have been designed and evaluated in multiple regions, and many of them were effective in improving glycemic and other metabolic parameters [4, 5].
India has nearly 73 million people affected by diabetes, and by 2045 the numbers are projected to increase to 134 million, which will make it the nation with the highest number globally [1]. Given the high burden of T2DM and its complications in India, effective programs are required that can improve outcomes over and above usual care advice on diet and exercise [2, 3]. However, most of the programs have focused on prevention, and little evidence, if any, exists on such programs for management of diabetes in India [4,5,6,7,8]. In an earlier program, called D-CLIP, participants received a culturally modified diabetes prevention program in a face-to-face intervention, and this resulted in a 32% relative risk reduction of T2DM [6]. A reality video-based lifestyle program based on D-CLIP was developed (12 modules of 15–20 min) and delivered through mobile devices [9]. This was recently tested in individuals at high risk of developing diabetes in India and was found to be effective [10]. In our study, we aimed to evaluate the same program (but compressed 12 modules into 4 videos), which were then delivered in person as an interactive sessions, in individuals with T2DM who had poor glycemic control. To the best of our knowledge, this is the first such VBLEP evaluated in individuals with T2DM from India.
Methods
Settings and Study Design
This randomized controlled trial (RCT) was performed from June 2017 to October 2018 at the All India Institute of Medical Sciences (AIIMS), New Delhi, a tertiary care hospital in North India. The AIIMS ethics committee approved the study, and written informed consent was obtained from all participants. The study was done as per the ethics delineated in the Helsinki Declaration.
Patient Identification, Recruitment, Inclusion, and Exclusion Criteria
Individuals with T2DM, diagnosed at ≥ 30 years of age and willing to participate in the study as per protocol, were identified from the outpatient clinic of the Department of Endocrinology and Metabolism, AIIMS. Those with HbA1c 7.5–10% and on stable oral glucose-lowering agents for the last 3 months were eligible. Patients with stage 4 and 5 chronic kidney disease, chronic liver disease, cancer, terminal illness, severe non-proliferative diabetic retinopathy, proliferative diabetic retinopathy or macular edema, any cardiovascular event requiring hospitalization in the last 6 months or with active diabetic foot ulcer were excluded. In addition, patients on insulin or other parenteral diabetes therapies were excluded.
Procedure on Day of Testing and Measurements
Participants were advised to fast for at least 10 h for the first visit for the evaluation of the fasting plasma glucose, lipid profile, and HbA1c in the morning. Additional information such as the demographics and relevant medical and treatment history was collected. Weight, height, waist circumference, and blood pressure were recorded in fasting state using standard methods [11]. Serum total cholesterol, triglyceride, and high-density lipoprotein (HDL) cholesterol levels and glucose levels were measured directly using an automated biochemistry analyzer (Cobas Integra 400 plus; Roche Diagnostics, Mannheim, Germany). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation, except in cases with serum triglyceride levels ≥ 400 mg/dl (4.5 mmol/l). Blood for HbA1c was measured by Toshiba G-8 (Tosoh Corp., Japan).
Intervention and Usual Care Details
Video-based lifestyle education program (VBLEP): The curriculum used in the D-CLIP diabetes prevention study was converted into a 12-week video-based reality television series [available as an app (free and commercial) from https://apps.apple.com/in/app/habits-diabetes-coach/id1016026169] [6, 9]. We further adapted and consolidated this content to create four sessions, delivered at weekly intervals, with permission from the developers of the reality television program (Janacare Solutions Private, Ltd, Bengaluru) (details provided in Table 1).
The participants in the usual care arm were prescribed dietary advice by a registered dietician and 30 min of walking at a speed of 5–6 km/h for at least 5 days a week.
We also evaluated yoga as an additional arm in this RCT to gain additional insights into this increasingly popular means of exercise in India. We used the same usual care group, against which each of the two lifestyle intervention arms was compared. This design benefited us as fewer participants (25% less) had to be recruited (using same usual group) with potential savings in cost and time. In this article we present and discuss the results of a video-based lifestyle education program compared with usual care.
Outcome Measures
The primary outcome measure was the difference in change in mean HbA1c between groups. Secondary outcome measurements were change in fasting plasma glucose (FPG), weight, body mass index (BMI), waist circumference, blood pressure, and lipid parameters.
Sample Size Calculation
With the following assumptions, mean HbA1c of 8.8, SD 0.6 [values from baseline parameters of individuals with HbA1c 7.5–10% enrolled at the Department of Endocrinology & Metabolism, AIIMS in the Delhi centre of INDEPENDENT (Integrating DEPrEssioN and Diabetes treatment) Study] [12], and a delta of 0.5 between the intervention and usual care arm, the estimated sample size was 36 in each arm. The assumed power and alpha were 90% and 2.5%, respectively. The alpha error adjusted for multiple testing as the study intended to compare two different lifestyle intervention programs vs. usual care (see details at trial registration site CTRI registration no.: CTRI/2017/05/008564).
Randomization, Allocation Concealment, and Blinding
Patients were randomized in a 1:1 ratio using block randomization with varying block size by computer-generated random numbers. Allocation concealment was done by using sequentially numbered opaque sealed envelopes. Since the intervention was apparent, the trial was open-labeled and non-blinded. However, end point outcome assessment was done by a person who was not aware of the patient group to remove bias.
Statistical Analysis
Stata 12.0 (College Station, TX, USA) was used for statistical analysis. Pearson chi-square test was used to compare qualitative baseline variables among the groups. Quantitative baseline variables were compared using student’s t-test or wilcoxan rank sum test (as appropriate). Intention-to-treat and per protocol analysis was done for primary outcome. The difference in change in secondary outcomes in the two groups was assessed by per protocol analysis. The effect of VBLEP on primary and secondary outcomes compared with the usual care group was analyzed using linear regression analysis. The adjustment for the baseline serum triglyceride level was done in adjusted analysis as it was different at baseline between the two arms. The predictors associated with favorable outcome (difference in HbA1c ≥ 0.5% from baseline) were determined using logistic regression analysis, and the results are expressed as odds ratio (95% CI). The results are reported as the difference in changes between the two groups (95% CI). Data are presented as number (%), mean ± SD, or median (q25–q75) as appropriate. p < 0.025 was considered statistically significant.
Results
Baseline Characteristics
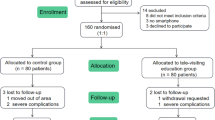
A total of 81 individuals were randomized, 41 participants in the usual care arm and 40 in the VBLEP arm. The participants (n = 81, women 48%) had mean (± SD) age of 50.1 (± 9.4) years, duration of diabetes 8.1 (± 6.0) years, HbA1c 8.5 ± 0.7% (68.87 ± 7.56 mmol/mol), and BMI (± SD) 27.7 ± 4.8 kg/m2. Family history of diabetes in first-degree relatives was present in 66.7%; 42.0% and 39.5% were on blood pressure and lipid-lowering medications, respectively. Thirty individuals (37%) were taking > 2 oral glucose-lowering agents. The main difference between the two study groups was in serum triglyceride level (p = 0.037), which was adjusted during per protocol analysis (Table 2). Of the 81 participants, one from the usual care arm and two from the VBLEP were lost to follow-up (Fig. 1, consort diagram). Adherence was defined as 75% attendance to intervention (attending three out of four video sessions). Of the 40 participants, 36 (90%) were adherent to the intervention.
Effect on Primary Outcome Measure
On intention-to-treat analysis there was a significant difference [0.64% 95% CI (0.14, 1.14); 7.0 mmol/mol 95% CI (1.50, 12.50), p = 0.013] in HbA1c in favor of VBLEP compared with usual care, which remained significant in adjusted analysis (Table 3). The results remained significant even after adjustment for triglycerides, which was different at baseline in two groups. On further analysis on per protocol, the difference between the two arms increased further, and the results were statistically significant.
On further analysis, 6 (15%) participants from usual care and 15 (39.5%) from VBLEP had HbA1c reduction ≥ 1%, (p = 0.012). The reduction in HbA1c by ≥ 0.5% was observed in 65.8% of participants in the VBLEP compared with 37.5% in the usual care arm.
Effect on Secondary Outcome Parameters
There was a significant reduction in weight and BMI at 4 months in favor of VBLEP compared with usual care. There was also favorable reduction in fasting plasma glucose and waist circumference at 4 months in participants randomized to VBLEP (Table 4).
Predictors of Favorable Outcome (Difference in HbA1c ≥ 0.5% from Baseline)
Participants who were employed, had a family history of diabetes, had no diabetes-related complications, and were in the VBLEP group had higher odds of having favorable HbA1c reduction (≥ 0.5%, combined analysis of both groups). In addition, female subjects and those taking > 2 oral glucose-lowering agents, anti-hypertensive therapy, or lipid-lowering therapy had higher favorable outcomes (Table 5).
Discussion
In this trial, we evaluated the effect of a VBLEP delivered over four sessions in participants with T2DM having poor glycemic control. We found a clinically relevant reduction in HbA1c (≥ 1% decrease) in many individuals (39.5%) and statistically significant mean reduction in HbA1c in the VBLEP (video-based lifestyle education program) compared with usual care. This suggests that a video-based structured real-life character-based education program, when delivered in person, may have greater benefits. There was a significant reduction in weight and BMI observed in the VBLEP compared with the usual care group.
The study found a significant difference [0.6% 95% CI (0.1, 1.1); 7.0 mmol/mol 95% CI (1.5, 12.5), p = 0.013] in HbA1c in favor of the VBLEP compared with usual care on intention-to-treat analysis, which remained significant in adjusted analysis. Furthermore, reduction in HbA1c by > 0.5% and 1% was seen in 65.8% and 39.5% of participants, respectively, in the VBLEP arm. The corresponding figures observed in the usual care arm were 37.5% and 15%, respectively.
Odgers-Jewell et al. recently reported findings on the effectiveness of group-based self-management education in T2DM [5]. The mean duration of diabetes (approximately 9 years) was similar to that in our study with greater HbA1c reduction (by 0.3%) in the intervention group. The benefits were greater in individuals on oral glucose-lowering agents and in those who had a baseline HbA1c > 7%. Delivery of the intervention by health care professionals from a single discipline in secondary/tertiary care hospitals was found to have the greatest benefits. These may be some of the reasons underlying the benefits reported in our study. Our study enrolled participants who were only on oral glucose-lowering agents for diabetes management and had baseline HbA1c ≥ 7.5% to ≤ 10%. The intervention was delivered in groups with 3–5 participants by a physician (Uttio Gupta) doing his fellowship in endocrinology at a tertiary care hospital. The session content was delivered in the form of real character-based videos covering education related to lifestyle modification for diabetes management. In between and after the videos, the endocrine fellow carried out reinforcements and discussions on important points/queries. In per protocol analysis for secondary outcomes, there was weight reduction in favor of the VBLEP compared with usual care. This effect was similar to that reported in the meta-analysis [8]. The effect on lipid and blood pressure parameters was not significant.
We also evaluated the factors associated with favorable outcome defined by HbA1c reduction of ≥ 0.5% from baseline at 4-month follow-up, irrespective of group allocation. The individuals who had lower education levels, were females, had a family history of diabetes, were on > 2 oral glucose-lowering agents, were taking anti-hypertensive treatment, and were on lipid-lowering therapy had higher odds of favorable outcome. The presence of diabetes-related complications was associated with a less favorable HbA1c outcome.
To the best of our knowledge according to the literature review, this is the first RCT from South Asia evaluating a real character-based television program for the management of diabetes. Using a video-based form of intervention delivered interactively adds to its strength. This study has provided preliminary insights into the magnitude of the effect that this form of intervention can have. A key limitation of our study was lack of long-term follow-up data since it was piloted for only 4 months. Long follow-up periods may have different implications for the outcomes and sustainability of benefits.
Conclusions
To conclude, a video-based lifestyle education program is an effective strategy to achieve better glycemic control in people with T2DM in addition to usual care. That nearly 40% of participants had > 1% improvement in HbA1c suggests that this simple technology-based education program made up of four videos can be easily incorporated in routine clinical practice.
References
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res ClinPract. 2018;138:271–81.
Hills AP, Misra A, Gill JMR, Byrne NM, Soares MJ, Ramachandran A, Palaniappan L, Street SJ, Jayawardena R, Khunti K, Arena R. Public health and health systems: implications for the prevention and management of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018;6:992–1002.
Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016;12:357–70.
Huang XL, Pan JH, Chen D, Chen J, Chen F, Hu TT. Efficacy of lifestyle interventions in patients with type 2 diabetes: a systematic review and meta-analysis. Eur J Intern Med. 2016;27:37–47.
Odgers-Jewell K, Ball LE, Kelly JT, Isenring EA, Reidlinger DP, Thomas R. Effectiveness of group-based self-management education for individuals with type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027–39.
Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and met form in prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49:289–97.
Ramachandran A, Snehalatha C, Ram J, Selvam S, Simon M, Nanditha A, Shetty AS, Godsland IF, Chaturvedi N, Majeed A, Oliver N, Toumazou C, Alberti KG, Johnston DG. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013;1:191–8.
Weber MB, Ranjani H, Staimez LR, Anjana RM, Ali MK, Narayan KMV, Mohan V. The stepwise approach to diabetes prevention: results from the D-CLIP randomized controlled trial. Diabetes Care. 2016;39:1760–7.
Muralidharan S, Mohan V, Anjana RM, Jena S, Tandon N, Allender S, Ranjani H. Mobile health technology (mDiab) for the prevention of type 2 diabetes: protocol for a randomized controlled trial. JMIR Res Protoc. 2017;6:e242.
Muralidharan S, Ranjani H, Mohan Anjana R, Jena S, Tandon N, Gupta Y, Ambekar S, Koppikar V, Jagannathan N, Allender S, Mohan V. Engagement and weight loss: results from the mobile health and diabetes trial. Diabetes Technol Ther. 2019;21:507–13.
Goyal A, Gupta Y, Kalaivani M, Sankar MJ, Kachhawa G, Bhatla N, Gupta N, Tandon N. Concordance of glycaemic and cardiometabolic traits between Indian women with history of gestational diabetes mellitus and their spouses: an opportunity to target the household. Diabetologia. 2019;62:1357–65.
Kowalski AJ, Poongothai S, Chwastiak L, Hutcheson M, Tandon N, Khadgawat R, et al. The INtegratingDEPrEssioN and Diabetes treatmENT (INDEPENDENT) study: design and methods to address mental healthcare gaps in India. Contemp Clin Trials. 2017;60:113–24.
Acknowledgements
The authors especially thank Janacare Solutions Private, Ltd., Bengaluru, for providing the content of the reality television-based prevention program used in the VBLEP intervention arm. The content of the reality television-based prevention program was adapted from the D-CLIP curriculum developed by Madras Diabetes Research Foundation (MDRF). The authors also thank Ankit Rajput, who provided technical assistance in carrying out this study. We are grateful to all the participants who gave their valuable time for this study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
UG conducted the research and wrote the article. YG contributed to planning the research, interpreting the results, and drafting and revising the manuscript. NT contributed to the planning and design of the research, interpretation of results, and conception of the article. KM analyzed and interpreted the data. All authors were involved in the initial planning, execution of the study, critical review of the manuscript, and final approval of manuscript.
Prior Presentation
This study was presented as a poster at ESICON (Annual Conference of the Endocrine Society of India), 15–18 November 2018. The abstract was published in IJEM Supplement 1, November 2018 [http://www.ijem.in/temp/IndianJEndocrMetab22717-3287394_090753.pdf].
Disclosures
Uttio Gupta, Yashdeep Gupta, Divya Jose, Kalaivani Mani, Viveka P. Jyotsna, Gautam Sharma, and Nikhil Tandon have nothing to disclose.
Compliance with Ethics Guidelines
The AIIMS ethics committee approved the study, and written informed consent was obtained from all participants. The study was done as per the ethics delineated in the Helsinki Declaration.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11591100.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Gupta, U., Gupta, Y., Jose, D. et al. Effectiveness of a Video-Based Lifestyle Education Program Compared to Usual Care in Improving HbA1c and Other Metabolic Parameters in Individuals with Type 2 Diabetes: An Open-Label Parallel Arm Randomized Control Trial (RCT). Diabetes Ther 11, 667–679 (2020). https://doi.org/10.1007/s13300-020-00769-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00769-2