Abstract
The ever-increasing number of drugs available to treat type 2 diabetes and the complexity of patients with this condition present a constant challenge when it comes to identifying the most appropriate treatment approach. The more recent glucagon-like peptide-1 receptor agonists (GLP-1RAs) are non-insulin injectable options for the management of type 2 diabetes. Effective at improving glycaemic control with a low intrinsic risk of hypoglycaemia and the potential for weight reduction, this agent class is an important addition to the prescribing armamentarium. However, understanding their place in therapy may prove confusing for many primary care practitioners, especially given the common belief that ‘injectables’ are a last-resort treatment option, which puts them at risk of being niched alongside insulin. This review summarises the clinical evidence for GLP-1RAs and how they compare to other glucose-lowering agents in managing type 2 diabetes. It also provides practical and case-driven opinions and recommendations on the optimal use of GLP-1RAs by discussing important patient factors and clinical considerations that will help to identify those who are most likely to benefit from this class of agents.
Funding: Eli Lilly Australia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the availability of an increasing number of agents for treating type 2 diabetes mellitus (T2DM), as many as half of all T2DM patients are failing to meet their glycaemic goals [1]. This paradox may reflect a treatment landscape that appears complex and confusing, making it difficult for practitioners to identify the right drug for the right patient. Moreover, clinical inertia, including a reluctance to use injectable therapies, may also be compromising optimal treatment selection and thus clinical outcomes [2, 3].
GLP-1RAs are a relatively new class of injectables that are effective at reducing HbA1c, have a low risk of hypoglycaemia when given as monotherapy or in the absence of sulphonylureas (SU) or insulin, and have the potential for weight loss—key considerations when treating a typical obese patient with T2DM [4]. In addition, individual GLP-1RAs have been developed to overcome common barriers to self-injection by offering a lower injection burden (i.e. weekly vs. daily injections) and devices with a ‘hidden’ pre-attached needle (e.g. dulaglutide) [5, 6].
Nonetheless, current clinical practice in managing T2DM using GLP-1RAs is likely to vary worldwide due to differences in healthcare systems and their accessibility, in the availability and affordability of medications, as well as in country-specific reimbursement policies. For instance, Australia differs to other regions (such as the USA) where patients are responsible for almost all of their healthcare costs in having a publicly funded healthcare insurance system that provides a rebate for doctor and specialist visits, blood tests and X-rays, although it should be noted that the rebate does not always cover the full cost of medical services, and patients incur ‘out-of-pocket’ expenses. Also, Australia, the USA and the UK differ in the GLP-1RAs that have received regulatory approval. Among those currently available in Australia, not all are eligible for government reimbursement. Adding to the complexity are the criteria for a subsidised GLP-1RA, which can limit the choice of additional or add-on therapies; e.g. at present, the government will not subsidise a GLP-1RA if given with an insulin. This therefore affects the prescribing patterns of GLP-1RAs in Australia.
In other regions, e.g. Asia or the Middle East, access to and the use of GLP1-RAs are primarily driven by whether the patient can afford it.
Five GLP-1RAs are available in Australia (dulaglutide, exenatide BD, exenatide QW, liraglutide, and lixisenatide), one is undergoing regulatory approval (semaglutide), and three are currently available under the government’s reimbursement scheme (dulaglutide, exenatide BD, and exenatide QW). The aim of this article is to explore their clinical rationale, assess where they fit in the current guideline approach and identify which patients may derive benefit from their use. This will allow practitioners to be better informed about why, when and how to treat T2DM with GLP-1RAs appropriately. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Clinical Rationale for GLP-1RAs in T2DM
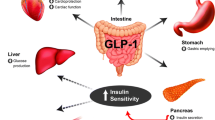
It is well recognised that T2DM is a complex and multifactorial disorder attributable to the eight underlying pathophysiological defects labelled the ‘ominous octet’ [7]. Involving multiple organs, these abnormalities collectively drive the clinical manifestation of the disease (i.e. hyperglycaemia) [7]. The importance of targeting the underlying pathophysiology of T2DM rather than focusing management on reducing plasma glucose levels, as is currently recommended, has recently been debated [8]. It was argued that therapeutic guidelines fail to achieve sustained HbA1c reductions and therefore treatment should be based on addressing pathophysiological defects [8]. In this context, GLP-1RAs are able to target six of these biological abnormalities, whereas other agents such as metformin target mainly one (Fig. 1) [7, 8].

Adapted from Defronzo [7]
Pathophysiological targets of GLP-1RAs in T2DM.
Fundamentally, GLP-1RAs work by stimulating insulin secretion and inhibiting glucagon secretion in a glucose-dependent manner [9], which explains their associated low risk of hypoglycaemia. Importantly, GLP-1RAs are able to improve β-cell sensitivity to glucose and have the ability to improve the glycaemic profile indirectly by delaying gastric emptying, inhibiting hepatic glucose production and suppressing appetite, thereby promoting weight loss [9,10,11].
Guideline Recommendations on GLP-1RAs
When and Where Do GLP-1RAs Fit into Current Management?
As different classes of drugs target different aspects of T2DM via distinct mechanisms of action, a combination of treatments is generally more effective at maintaining glycaemic control than monotherapy over the long term [12]. Indeed, Australian guidelines recommend GLP-1RAs as an add-on option to metformin from the second-line setting (Fig. 2) [13, 14], dispelling the common notion that injectable therapies are a ‘last resort’ or restricted only to those individuals who fail to respond to maximal doses of oral agents. These guidelines, however, do not give a preference regarding which of the second-line agents—which also include sulfonylureas (SU), dipeptidyl peptidase-4 inhibitors (DPP-IV), sodium-glucose cotransporter 2 inhibitors (SGLT-2i), insulin, thiazolidinediones (TZD) and acarbose—should be added to optimised metformin [13, 14]. In contrast, recommendations for treatment selection in the second-line setting have been outlined in international guidelines, such as the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) guidelines, which inform choice of treatment according to an individual’s CV and chronic kidney disease (CKD) risk [15]. Given the increasing availability of CV safety outcomes data for glucose-lowering medications, it has become apparent that certain agents within the GLP-1RA and SGLT-2 inhibitor classes reduce the risk of CV events in those with high-risk CV or CKD [16,17,18,19,20,21]. With respect to GLP-1RAs, liraglutide and semaglutide have demonstrated a reduction in the risk of major adverse cardiovascular events (MACE) [16, 17], and, while yet not published, early reports for dulaglutide indicate CV benefits in patients with a wide range of CV risk [18]. It is important to note that while GLP-1RAs have demonstrated CV safety, not all result in an actual reduction in CV adverse events, such as exenatide QW and lixisenatide, both of which were reported to be non-inferior to placebo for MACE [22, 23].
Treatment selection should therefore take into consideration efficacy, adverse event profile, hypoglycaemia risk, weight control, presence of comorbidities and CV/CKD risk, as well as cost to patient [13,14,15, 24].
Case Scenario to Guide Second-Line Treatment Selection
A 52-year-old man with a three-year history of T2DM presents for a review. His current HbA1c is 8.0% (64 mmol/mol) and he weighs 109.7 kg (BMI: 35.1 kg/m2), which have increased since his last review a year ago. He is receiving a maximal daily dose of metformin extended release (2000 mg; nocte). He also has a history of hypertension and dyslipidaemia that are being successfully managed with amlodipine, low-dose aspirin and atorvastatin. He works in construction in a hot environment.
Suboptimal glycaemic control and increasing weight gain—major risk factors for blood glucose and CV complications—are key concerns for this patient. How would the addition of a GLP-1RA compare to other second-line add-on therapies in improving management of the T2DM?
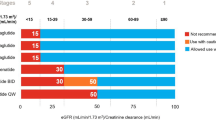
A review of head-to-head clinical trials comparing key outcomes of using GLP-1RAs (dulaglutide, exenatide BD, exenatide QW, liraglutide or lixisenatide) with those of using DPP-IV (sitagliptin), SU (glimepiride; glibenclamide), TZD (rosiglitazone; pioglitazone) and insulin (insulin aspart; insulin glargine) on a background of metformin monotherapy is outlined in Table A below [25]. Note, no head-to-head studies of GLP-1RAs and SGLT-2 inhibitors or acarbose are available.
Table A: GLP-1RAs versus other glucose-lowering agents: key specific features that impact treatment choice
HbA1c reductions (%) | Body weight changes (kg) | GI side effects | Hypoglycaemiaa rate | |
|---|---|---|---|---|
GLP-1RA vs. OADs | ||||
0.7 to 1.8 vs. 0.3 to 0.9b | − 2.3 to − 3.7 vs. − 0.8 to − 1.8f | Greater or similar to DPP-IV | Minor episodes low for both No difference between classes Severe episodes rare* | |
0.4 to 1.5 vs. 0.2 to 1.8c | − 2.8 to − 8.0 vs. + 0.7 to + 4.3f | Greater than SU | Minor episodes lower than SUs No severe episodes reported** | |
− 0.9 to − 1.5 vs. − 1.0 to − 1.2d | − 2.3 to − 2.8 vs. + 1.5 to + 2.8f | Greater than TZD | Minor episodes slightly greater or similar to TZD Severe episodes rare† | |
GLP-1RA vs. other injectables | ||||
− 1.0 to − 1.8 vs. − 0.9 to − 1.9e | − 2.5 to − 4.1 vs. + 0.8 to + 2.3f | Greater than insulins | Minor episodes generally lower‡ Severe episodes rare§ | |
Given that there are few head-to-head studies comparing glucose-lowering effects between different drug classes, and the difficulty involved in performing cross-study comparisons due to different patient characteristics and baseline HbA1c levels, there is widespread consensus that GLP-1RAs are more efficacious at lowering blood glucose than either DPP-IVs or SGLT-2 inhibitors. The expected glucose-lowering efficacy is an important consideration when changing from one glucose-lowering agent to another or when adding a new agent to existing therapy.
So, while DPP-IVs may offer a better gastrointestinal (GI) adverse event profile than GLP-1RAs, they are generally less effective at reducing HbA1C and have a lower potential for weight loss. SUs are as effective as GLP-1RAs in HbA1C reduction and have fewer GI effects, but they can lead to weight gain and also a higher risk of hypoglycaemia, which this patient needs to avoid. Similarly, TZDs are as effective as GLP-1RAs in HbA1C reduction, have fewer GI effects and may have a lower hypoglycaemia risk, but can cause weight gain.
Insulins lead to marked HbA1c reductions and have a lower risk of GI effects but can lead to weight gain and are generally associated with a higher risk of hypoglycaemia than GLP-1RAs, which would present significant occupational implications for this patient. While insulins and GLP-1RAs have been considered equally effective at reducing HbA1c [41, 42], a more recent meta-analysis reported that long-acting, once-weekly GLP-1RAs (exenatide and dulaglutide) achieve significantly greater reductions in HbA1c than basal insulins such as insulin detemir, insulin glargine and insulin degludec [43]. However, insulin was found to be more effective at reducing fasting plasma glucose but less effective at reducing postprandial glucose levels than GLP-1RAs [42, 44]. Several systematic reviews also indicated that GLP-1RAs are consistently effective at reducing body weight [25, 41, 42, 44], supporting the nonglycaemic role of this agent class [44], as opposed to the weight gain that commonly accompanies insulin treatment [44]. Furthermore, the risk of hypoglycaemia—a major challenge when using insulin—is reportedly lower with GLP-1RAs, given their glucose-dependent mechanism of modulating insulin release and glucagon suppression [25]. Alongside these benefits of GLP-1RAs, however, is an increased propensity for GI adverse events, notably nausea, when compared with insulin. Table 1 outlines these comparisons further.
Given that weight control and a low risk of hypoglycaemia are key considerations for this patient, the addition of a GLP-1RA may be the most appropriate choice. This selection is further supported by the CV outcomes data for specific GLP-1RAs, which point to benefits in reducing the risk of MACE in at-risk individuals [16,17,18]. The most appropriate GLP-1RA to use will ultimately be dictated by the device and how it aligns with the patient’s needs, as well as associated costs and the reimbursement status of the drug options.
Individualising Care: Choosing the Right GLP-1RA for the Right Patient
Not All GLP-1RAs are Made Equal
GLP-1RAs are often differentiated according to structure and duration of action (Table 2) [9, 24, 45,46,47,48]. In terms of structure, several GLP-1RAs have been developed based on the naturally occurring protein exendin-4 (from the Gila monster, a lizard found in New Mexico and Arizona), which shares 53% homology with native (human) GLP-1 [49,50,51]. Other GLP-1RAs have exploited the native GLP-1 molecule with 90–97% homology and modifications to resist degradation by the enzyme DPP-IV [35, 48].
GLP-1RAs can also be differentiated according to their duration of receptor activation. Short-acting agents undergo renal clearance, so they require once-daily (lixisenatide) or twice-daily (exenatide BD) dosing. Long-acting agents, on the other hand, provide continuous receptor activation due to structural modifications that slow their absorption, reduce the rate of renal clearance or extend their half-lives [52, 53]. These GLP-1RAs include once-weekly dulaglutide, exenatide and semaglutide (yet to be approved in Australia) and once-daily liraglutide (Table 2). Differences in the duration of action may also account for their differential effects on fasting and postprandial glucose [54]. For instance, short-acting GLP-1RAs are more strongly associated with delayed gastric emptying than long-acting agents, leading to a greater impact on postprandial glucose. In contrast, the persistent effects of longer-acting GLP-1RAs offer 24-h glucose control, including fasting glucose [53]. As a result, patients with largely postprandial hyperglycaemia are more likely to benefit from treatment with short-acting GLP1-RAs as opposed to individuals with predominantly fasting hyperglycaemia, who would derive greater benefit from long-acting agents [52].
In terms of tolerability, GLP-1RAs are commonly associated with GI side effects, which are often dose-dependent and time-limiting [55]. However, long-acting GLP-1 RAs appear to cause less nausea and vomiting but more diarrhoea than short-acting agents [56]. The frequency of these events is lower with once-weekly GLP-1RAs compared to once-daily or twice-daily GLP-1RAs (Table 2) [57]. Of note, a small number of cases of acute pancreatitis associated with GLP-1RA use have been reported in clinical trials: 38 cases in 17,775 patient-years of exposure compared with 9 events in 5863 patient-years of exposure with the comparator treatment. Resulting pooled event rates were 2.1 and 1.5 per 1000 patient-years of exposure, respectively, and the OR was 1.39 (95% CI 0.67, 2.88), suggesting a slightly elevated risk [58]. In a separate meta-analysis, a significantly increased risk of cholelithiasis (OR 1.30; 95% CI 1.01–1.68; p = 0.041) but not pancreatitis or pancreatic cancer was reported for GLP-1RAs compared with comparator treatments [59]. A more recent analysis using cardiovascular outcome trials further indicated no excess risk of either acute pancreatitis [Peto OR 0.89 (95% CI 0.63, 1.27)] or pancreatic cancer [Peto OR 0.84 (95% CI 0.53, 1.35)] with GLP-1RAs vs. placebo when added to the standard of care [60]. An increased risk of diabetic retinopathy has been reported in a clinical trial of semaglutide, which has been hypothesised to be related to the magnitude and rapidity of glucose lowering [61]; this complication does not appear to be a class-driven effect.
Tackling Adherence Challenges One GLP-1RA at a Time
T2DM management has shifted from a ‘one size fits all’ approach to a more holistic, patient-centred approach that aligns goals of management, such as glycaemic targets and risk factor control (e.g. weight or CV risk), with patient preferences and characteristics [62]. Involving the patient in treatment decisions also helps with adherence to therapy, which is a major challenge in T2DM. For instance, in an Australian study, approximately a third of patients with T2DM were found to have suboptimal adherence to their diabetes medication [63].
Strategies that address convenience in terms of simplicity of drug regimens, formulations and delivery devices have been shown to improve adherence [64]. Indeed, GLP-1RAs have evolved over time such that the frequency of dosing has decreased from twice-daily (exenatide, BD) [65], to once-daily (liraglutide, lixisenatide) [66, 67] to once-weekly (dulaglutide, exenatide QW, semaglutide) [5, 6], suggesting a higher adherence potential for the longer-acting agents (Table 2). Moreover, improvements in drug formulations (such as those which do not require reconstitution or dose titration) and injection devices with hidden and pre-attached needles, which together serve to reduce treatment complexity, may lead to a more positive injection experience for patients (Table 3) [68].
Indeed, lowering the regimen complexity and treatment burden has been shown to improve treatment satisfaction, which in turn plays an important role in supporting adherence to medication [69]. Data on patient-reported outcomes for individual GLP-1RAs indicate high treatment satisfaction rates with the long-acting agents (Table 3) [69,70,71,72,73,74], while dulaglutide was associated with higher adherence and persistence rates than exenatide QW and liraglutide [75].
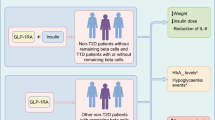
Given the many factors that come into play when deciding on the most appropriate choice of treatment for T2DM in an individual patient, in the context of GLP-1RAs, a clinical decision algorithm that incorporates author opinion is proposed; see Fig. 3 [5, 6, 24, 50].
Practical Considerations for GLP-1RA Use
As GLP-1RAs are injectables, comparisons of GLP-1RAs with insulins are inevitable. However, the practicalities of initiating GLP-1RAs are far less complicated, largely due to the simpler injection devices involved. For those patients who express concerns about injections or lack confidence with self-injections, it would be appropriate for the primary practitioner to demonstrate the ease with which these devices can be used. If needle phobia is a particular issue, discussion of options that have the needle hidden and/or have a pre-attached needle, which require minimal handling by the patient, could be considered.
While most GI side effects (nausea, vomiting and diarrhoea) caused by GLP-1RAs are mild to moderate and short-lived [5, 6, 50], they can be minimised by recommending simple measures such as eating smaller meals, stopping eating as soon as the patient feels full, and injecting at mealtimes. Some GLP-1RAs devices have the ability to easily adjust the dose in response to intolerance (e.g. the liraglutide pen allows for a 0.6-mg dose adjustment) [66, 76].
Diabetes educators and/or practice nurses with a specialty in diabetes are an important point of contact for patient education; such services are particularly valuable to primary practitioners who are time-constrained.
Conclusions
GLP-1RAs are effective at improving glycaemic control and, by virtue of their mechanism of action, have a low risk of hypoglycaemia combined with the potential for weight loss. Considering that many patients with T2DM are obese, these agents represent important options among the current therapeutic arsenal. From a practical point of view, different GLP-1RAs offer different dosing and device experiences, allowing practitioners to tailor treatment according to the needs of the individual patient.
References
Michaelides C. Mapping glycaemic control over Australia. Changing Diabetes Dashboard. 2012. http://glycomatetools.com/changingdiabetes/. Accessed 26 Apr 2019.
Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–7.
Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes. 2002;26(Suppl 3):S18–24.
Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–16.
Eli Lilly and Company. Trulicity product information. 2017. http://www.medicines.org.au/files/lyptruli.pdf. Accessed 28 Apr 2019.
AstraZeneca. Bydureon product information. 2015. http://www.medicines.org.au/files/appbydur.pdf. Accessed 28 Apr 2019.
DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
Abdul-Ghani M, DeFronzo R. Is it time to change the type 2 diabetes treatment paradigm? Yes! GLP-1 RAs should replace metformin in the type 2 diabetes algorithm. Diabetes Care. 2017;40:1121–7.
Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–42.
Chang AM, Jakobsen G, Sturis J, et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52:1786–91.
Bunck MC, Corner A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2041–7.
Cahn A, Cefalu WT. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care. 2016;39(Suppl 2):S137–45.
Gunton JE, Cheung NW, Davis TM, Zoungas S, Colagiuri S, Australian Diabetes Society. A new blood glucose management algorithm for type 2 diabetes: a position statement of the Australian Diabetes Society. Med J Aust. 2014;201:650–3.
The Royal Australian College of General Practitioners. General practice management of type 2 diabetes: 2016–18. East Melbourne: RACGP; 2016.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701.
Marso SP, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Eli Lilly and Company. Press release. Trulicity® (dulaglutide) demonstrates superiority in reduction of cardiovascular events for broad range of people with type 2 diabetes. 2018. https://investor.lilly.com/news-releases/news-release-details/trulicityr-dulaglutide-demonstrates-superiority-reduction. Accessed 19 May 2019.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Eng J Med. 2017;377:644–57.
Raz I, Mosenzon O, Bonaca MP, et al. DECLARE-TIMI 58: participants’ baseline characteristics. Diabetes Obes Metab. 2018;20(5):1102–10.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39.
Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57.
Kalra S. Choosing appropriate glucagon-like peptide 1 receptor agonists: a patient-centered approach. Diabetes Ther. 2014;5(1):333–40.
Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes. 2017;10:123–39.
Bergenstal RM, Wysham C, Macconell L, DURATION-2 Study Group, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431–9.
Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–58.
Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17(9):849–58.
Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65(4):397–407.
Van Gaal L, Souhami E, Zhou T, Aronson R. Efficacy and safety of the glucagon-like peptide-1 receptor agonist lixisenatide versus the dipeptidyl peptidase-4 inhibitor sitagliptin in young (< 50 years) obese patients with type 2 diabetes mellitus. J Clin Transl Endocrinol. 2014;1(2):31–7.
Derosa G, Maffioli P, Salvadeo SA, et al. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol Ther. 2010;12(3):233–40.
Derosa G, Putignano P, Bossi AC, et al. Exenatide or glimepiride added to metformin on metabolic control and on insulin resistance in type 2 diabetic patients. Eur J Pharmacol. 2011;666(1–3):251–6.
Gallwitz B, Guzman J, Dotta F, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 2012;379(9833):2270–8.
Nauck M, Frid A, Hermansen K, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15(3):204–12.
Nauck M, Frid A, Hermansen K, LEAD-2 Study Group, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care. 2009;32(1):84–90.
DeFronzo RA, Triplitt C, Qu Y, Lewis MS, Maggs D, Glass LC. Effects of exenatide plus rosiglitazone on beta-cell function and insulin sensitivity in subjects with type 2 diabetes on metformin. Diabetes Care. 2010;33(5):951–7.
D’Alessio D, Häring HU, Charbonnel B. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17(2):170–8.
Davies M, Heller S, Sreenan S, et al. Once-weekly exenatide versus once- or twice-daily insulin detemir: randomized, open-label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care. 2013;36(5):1368–76.
Diamant M, Van Gaal L, Guerci B, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol. 2014;2(6):464–73.
Gallwitz B, Böhmer M, Segiet T, et al. Exenatide twice daily versus premixed insulin aspart 70/30 in metformin-treated patients with type 2 diabetes: a randomized 26-week study on glycemic control and hypoglycemia. Diabetes Care. 2011;34(3):604–6.
Li WX, Gou JF, Tian JH, Yan X, Yang L. Glucagon-like peptide-1 receptor agonists versus insulin glargine for type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Curr Ther Res Clin Exp. 2010;71(4):211–38.
Abdul-Ghani MA, Williams K, Kanat M, Altuntas Y, DeFronzo RA. Insulin vs GLP-1 analogues in poorly controlled type 2 diabetic subjects on oral therapy: a meta-analysis. J Endocrinol Investig. 2013;36(3):168–73.
Singh S, Wright EE Jr, Kwan AY, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(2):228–38.
Wang Y, Li L, Yang M, Liu H, Boden G, Yang G. Glucagon-like peptide-1 receptor agonists versus insulin in inadequately controlled patients with type 2 diabetes mellitus: a meta-analysis of clinical trials. Diabetes Obes Metab. 2011;13(11):972–81.
Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists—available efficacy and safety data and perspectives for the future. Diabetes Obes Metab. 2011;13:394–407.
Kalra S, Baruah MP, Sahay RK, Unnikrishnan AG, Uppal S, Adetunji O. Glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes: past, present, and future. Indian J Endocrinol Metab. 2016;20(2):254–67.
Owens DR, Monnier L, Hanefeld M. A review of glucagon-like peptide-1 receptor agonists and their effects on lowering postprandial plasma glucose and cardiovascular outcomes in the treatment of type 2 diabetes mellitus. Diabetes Obes Metab. 2017;19(12):1645–54.
Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019;10:155.
Göke R, Fehmann HC, Linn T, et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650–5.
DeYoung MB, MacConell L, Sarin V, Trautmann M, Herbert P. Encapsulation of exenatide in poly-(d,l-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes Technol Ther. 2011;13:1145–54.
Quianzon CCL, Shomal ME. Lixisenatide—once-daily glucagon-like peptide-1 receptor agonist in the management of type 2 diabetes. Eur Endocrinol. 2012;8:12–7.
Uccellatore A, Genovese S, Dicembrini I, Mannucci E, Ceriello A. Comparison review of short-acting and long-acting glucagon-like peptide-1 receptor agonists. Diabetes Ther. 2015;6(3):239–56.
Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18(4):317–32.
Fineman MS, Cirincione BB, Maggs D, Diamant M. GLP-1 based therapies: differential effects on fasting and postprandial glucose. Diabetes Obes Metab. 2012;14:675–88.
Sun F, Chai S, Yu K, et al. Gastrointestinal adverse events of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Technol Ther. 2015;17(1):35–42.
Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting, and diarrhea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2016;19(3):336–47.
Horowitz M, Aroda VR, Han J, Hardy E, Rayner CK. Upper and/or lower gastrointestinal adverse events with glucagon-like peptide-1 receptor agonists: incidence and consequences. Diabetes Obes Metab. 2017;19(5):672–81.
Meier JJ, Nauck MA. Risk of pancreatitis in patients treated with incretin-based therapies. Diabetologia. 2014;57(7):1320–4.
Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab. 2017;19(9):1233–41.
Liu Y, Tian Q, Yang J, Wang H, Hong T. No pancreatic safety concern following glucagon-like peptide-1 receptor agonist therapies: a pooled analysis of cardiovascular outcome trials. Diabetes Metab Res Rev. 2018;34(8):e3061.
Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20(4):889–97.
Grant RW, Wexler DJ. Personalized medicine in type 2 diabetes: what does the future hold? Diabetes Manag (Lond, Engl). 2012;2(3):199–204.
Dhippayom T, Krass I. Medication-taking behaviour in New South Wales patients with type 2 diabetes: an observational study. Aust J Prim Health. 2015;21(4):429–37.
Giorgino F, Penfornis A, Pechtner V, Gentilella R, Corcos A. Adherence to antihyperglycemic medications and glucagon-like peptide 1-receptor agonists in type 2 diabetes: clinical consequences and strategies for improvement. Patient Prefer Adherence. 2018;12:707–19.
AstraZeneca. Byetta product information. 2017. http://www.medicines.org.au/files/appbyett.pdf. Accessed 28 Apr 2019.
Novo Nordisk. Victoza product information. 2017. http://www.novonordisk.com.au/content/dam/australia/affiliate/www-novonordisk-au/Health%20Care%20Professionals/Documents/Victoza%20pi7.pdf. Accessed 28 Apr 2019.
Sanofi–Aventis. Lyxumia product information. 2017. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2013-PI-01707-1&d=201904281016933. Accessed 28 Apr 2019.
Matfin G, Van Brunt K, Zimmermann AG, Threlkeld R, Ignaut DA. Safe and effective use of the once weekly dulaglutide single-dose pen in injection-naïve patients with type 2 diabetes. J Diabetes Sci Technol. 2015;9(5):1071–9.
Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48.
Best JH, Boye KS, Rubin RR, Cao D, Kim TH, Peyrot M. Improved treatment satisfaction and weight-related quality of life with exenatide once weekly or twice daily. Diabet Med. 2009;26(7):722–8.
Reaney M, Yu M, Lakshmanan M, Pechtner V, van Brunt K. Treatment satisfaction in people with type 2 diabetes mellitus treated with once-weekly dulaglutide: data from the AWARD-1 and AWARD-3 clinical trials. Diabetes Obes Metab. 2015;17(9):896–903.
Yu M, Van Brunt K, Varnado OJ, Boye KS. Patient-reported outcome results in patients with type 2 diabetes treated with once-weekly dulaglutide: data from the AWARD phase III clinical trial programme. Diabetes Obes Metab. 2016;18(4):419–24.
Davies M, Pratley R, Hammer M, Thomsen AB, Cuddihy R. Liraglutide improves treatment satisfaction in people with type 2 diabetes compared with sitagliptin, each as an add on to metformin. Diabet Med. 2011;28(3):333–7.
Schmidt WE, Christiansen JS, Hammer M, Zychma MJ, Buse JB. Patient-reported outcomes are superior in patients with type 2 diabetes treated with liraglutide as compared with exenatide, when added to metformin, sulphonylurea or both: results from a randomized, open-label study. Diabet Med. 2011;28(6):715–23.
Alatorre C, Fernández Landó L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19(7):953–61.
Brunton S. GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int J Clin Pract. 2014;68(5):557–67.
Acknowledgements
Funding
This review and the article processing charges were funded by Eli Lilly Australia. All authors had full access to the articles reviewed in this manuscript and take complete responsibility for the integrity and accuracy of this manuscript.
Medical Writing Assistance
Editorial assistance in the preparation of this article was provided by Dr. Beejal Vyas-Price of McCann Healthcare, Australia. Support for this assistance was funded by Eli Lilly Australia.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Roy Rasalam has received speaker honoraria from Eli Lilly. John Barlow has sat on advisory board panels and received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Mylan, Novartis and Sanofi/Aventis. Mark Kennedy has received honoraria for serving as a speaker for and advisory board member of Eli Lilly. Pat Phillips and Alan Wright have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8181860.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rasalam, R., Barlow, J., Kennedy, M. et al. GLP-1 Receptor Agonists for Type 2 Diabetes and Their Role in Primary Care: An Australian Perspective. Diabetes Ther 10, 1205–1217 (2019). https://doi.org/10.1007/s13300-019-0642-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-0642-2