Abstract
Introduction
In both randomized controlled trials and real-world studies, liraglutide has demonstrated glycemic and body weight benefits in patients with type 2 diabetes. However, persistence with diabetes medication can be challenging. This study compared glycated hemoglobin (HbA1c) and other outcomes in patients with type 2 diabetes who continued treatment with liraglutide for over 12 months with those who discontinued treatment earlier, in a real-life setting.
Methods
This is a retrospective study of adult patients with type 2 diabetes from Maccabi Healthcare Services in Israel, who initiated treatment with liraglutide from 2010 to 2015. Mean HbA1c and body weight change from initiation to after 24 months was compared between patients who received liraglutide for at least 12 months (“continuers”) and those who discontinued within the first year (“discontinuers”). Adjustment for HbA1c, body weight, and other potentially confounding factors was performed using 1:1 propensity score matching.
Results
The 3580 patients comprised 2695 continuers and 885 discontinuers; 882 patients per group were matched. A significant (p < 0.001) reduction in HbA1c (– 0.80% vs – 0.32%) was seen in continuers compared with discontinuers, despite higher insulin usage (70.2% vs 59.0%; p < 0.001), and a higher proportion of patients using ≥ 3 oral glucose-lowering drugs (20.6% vs 6.2%; p < 0.001) at 24 months among discontinuers. Mean body weight reduction was greater in continuers than discontinuers (3.57 vs 1.25 kg; p < 0.001).
Conclusion
In a real-world setting, persistent use of liraglutide was associated with good glycemic and body weight control.
Funding
Novo Nordisk Health Care AG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been shown to improve glycemic control by stimulating glucose-dependent insulin secretion and reducing glucagon secretion, to reduce food intake by slowing gastrointestinal motility and increasing satiety [1,2,3,4,5], and to reduce cardiovascular (CV) mortality/morbidity in patients with previous CV disease [6]. The safety and efficacy of liraglutide have been established in several randomized controlled trials, both as monotherapy and in combination with other oral glucose-lowering drugs (oGLDs) [7,8,9,10,11,12,13,14], and are supported by subsequent real-world studies [15, 16]. This robust clinical efficacy and safety profile was confirmed by a recent systematic literature review that included over 7400 patients treated with liraglutide in randomized and observational studies [16]. Liraglutide significantly reduced glycated hemoglobin (HbA1c) by 6 months from treatment initiation (baseline 7.2–9.8%; mean change: – 0.6% to – 2.3%) and reduced body weight (baseline 63.8–120 kg; mean change: – 1.3 to – 8.65 kg from baseline), with a higher baseline body mass index (BMI) associated with greater absolute weight loss [16]. Such improvements have been shown to occur alongside good adherence to and persistence with liraglutide for the study duration in the clinical trial setting [7,8,9,10,11,12,13,14]. Several studies have specifically addressed adherence to and persistence with liraglutide in patients newly initiating GLP-1RAs. These indicate that 29.0–60.0% of patients stay on liraglutide therapy for more than 1 year of treatment [17, 18]. In a review of published literature from a real-world setting, around half of those patients who initiated liraglutide were found to stop treatment during the first 2 years [16].
This study aimed to compare glycemic control at 24 months as well as other clinically important outcomes between patients with type 2 diabetes who continued treatment with liraglutide for ≥ 12 months and those who discontinued treatment earlier, in a real-life setting.
Methods
Study Design and Patients
This retrospective analysis of computerized clinical databases was carried out at Maccabi Healthcare Services (MHS). MHS covers ~ 25.0% of the population of Israel countrywide and includes ~ 2.1 million members, 160,000 (7.6%) of whom have documented diabetes, suggesting that it is representative of the population. The central computerized database stores data on members’ medical information, including medication [19]; specific inclusion criteria for the MHS diabetes registry are detailed elsewhere [20]. All procedures performed in studies involving human participants were in accordance with the local institutional review board of Bayit Balev Rehabilitation Hospital, Israel, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Individual patient-informed consent was not required because of the anonymized nature of the patient records.
All patient data from those who initiated liraglutide treatment between January 1, 2010, and February 28, 2015, were extracted for the study (n = 5932). Further inclusion criteria required that patients should be Maccabi members for at least 12 months prior to treatment initiation (index date) and until 24 months after treatment initiation, and should be > 18 years old at index date. Patients were required to have HbA1c measurements at baseline (defined as 180 days prior to the index date) and after 24 months from the index date within specified time windows. Patients who were defined in the diabetes registry as having type 1 diabetes, or were taking another GLP-1RA in the 6 months preceding the index date and until 24 months afterwards (including dispensing liraglutide after the discontinuation date), or who underwent bariatric surgery (12 months before/24 months after the index date) were excluded from the study.
Two groups of patients were defined within the cohort: “continuers,” who adhered to liraglutide treatment for ≥ 12 months, as measured by continuous liraglutide dispensed; and “discontinuers,” who stopped liraglutide before completing 12 months of treatment, as measured by a gap of ≥ 120 days between dispenses (after the refill date). Furthermore, the following subgroups were defined: patients above/below median HbA1c at baseline; insulin use at baseline; insulin treatment at 21–24 months after the index date; and liraglutide therapy at 24 months. Continuers were compared with discontinuers overall and within the subgroups.
Variable Definitions
For all laboratory measurements, baseline values were measured ≤ 180 days before the index date (most recent measurement used); early response values (HbA1c) were measured 90-270 days after baseline (the measurement closest to 180 days was used); and 24-month values were measured after 24 months (– 90/+ 180) days. The difference in change in body weight and BMI from baseline to 24 months between continuers and discontinuers was calculated. Low-density lipoprotein (LDL) cholesterol and triglyceride levels were measured at baseline and after 24 months. Values of > 300 mg/dL were recorded as 300 mg/dL for the analyses.
Oral and injectable diabetes medications used at baseline included those medications dispensed in the 180 days before baseline, and diabetes medication usage at 24 months included those medications dispensed 21–24 months after baseline. These included insulin (ATC codes: A10AB, A10AC, A10AD, A10AE), metformin (ATC codes: A10BA, A10BD07, A10BD08, A10BD10, A10BD11, A10BD15, A10BD16, A10BD20), sulfonylurea (ATC code: A10BB), meglitinides (ATC code: A10BX02), dipeptidyl peptidase-4 inhibitors (DPP-4i) (ATC codes: A10BH, A10BD07, A10BD08, A10BD10, A10BD11), sodium-glucose cotransporter-2 inhibitors (ATC codes: A10BX09, A10BX11, A10BX12, A10BD15, A10BD16, A10BD20, A10BK01, A10BK03), acarbose (ATC code: A10BF01), thiazolidinediones (ATC code: A10BG03), and GLP-1RAs (ATC codes: A10BX04, A10BX07, A10BX10, A10BX14, A10BJ01, A10BJ02, A10BJ03, A10BJ05). Medication use was defined as: insulin dispensed at 21–24 months (Y/N); using ≥ 3 oGLDs at 24 months (Y/N); numbers of oGLDs used at 21–24 months.
Hospitalizations 12–24 months after the index date were identified. Hospitalizations related to diabetes were defined according to the International Statistical Classification of Diseases and Related Health Problems (ICD-9) codes (diabetes mellitus with and without complications: 250.X; other specified hypoglycemia: 251.1; hypoglycemia, unspecified: 251.2; secondary diabetes mellitus with other specified manifestations: 249.8; diabetic retinopathy: 362.0; diabetic cataract: 366.41; polyneuropathy in diabetes: 357.2, and other abnormal glucose: 790.29). Other hospitalizations were defined as being unrelated to diabetes. Based on this, two hospitalization variables were defined: the number of overall hospitalizations in the 12–24 months after the index date; and the number of diabetes-related hospitalizations in the 12–24 months after the index date.
Duration of diabetes was defined as number of years in the MHS diabetes registry [21], which was based on diagnoses, medication dispensed, and laboratory measurements. Comorbidities reported within 12 months prior to the index date were defined according to the relevant disease registries (CV disease [22], cerebrovascular disease, chronic kidney disease [CKD], and hypertension [23]), or according to ICD-9 codes (dyslipidemia: 272.X; liver disease: 570.X–573.X; pancreatitis: 577.X; gallbladder disease: 575.X–576.X).
Statistical methods
The two groups were matched 1:1 using a propensity score based on baseline characteristics, including age, sex, baseline HbA1c, diabetes duration, BMI, and insulin use. Subgroups were rematched for subgroup analyses.
Descriptive statistics were reported for all baseline characteristics and clinical outcomes separately for continuers and discontinuers before and after propensity score matching. For continuous variables, mean ± standard deviation (SD) were reported. For categorical variables, n (%) were reported. The magnitude of the effect size for the change from baseline to follow-up measurement in key measures between continuers and discontinuers was calculated as follows [24]:
where the effect size was categorized as follows: 0.01 = very small, 0.2 = small, 0.5 = medium, 0.8 = large, 1.2 = very large, and 2 = huge.
For each variable, statistical comparisons between continuers and discontinuers were performed using Fisher’s exact test or the χ2 test for categorical variables, and the t test or the Wilcoxon rank-sum test for continuous variables. Analyses were carried out using two-tailed tests, and p values of less than 0.05 were considered statistically significant.
Propensity Scores
Propensity scores were developed using a multivariate logistic regression model where the dependent binary variable indicated whether the patient was a new user of liraglutide who adhered to liraglutide treatment for ≥ 12 months (= 1) or discontinued liraglutide before completing 12 months of treatment (= 0). Propensity scores estimated the probability of adherence to liraglutide treatment for ≥ 12 months or discontinuing liraglutide before completing 12 months of treatment, given the covariates in the model. Independent covariates at the index date were: age, sex, diabetes duration (≤ 2, 2–10, or > 10 years), HbA1c level, BMI (< 25, 25–30, 30–32.5, 32.5–35, 35–40, > 40 kg/m2, or missing), and use of insulin.
Patients were matched 1:1 using the “greedy matching” technique, which randomly selects a treated patient and matches them to the nearest untreated subject—in this case, continuers and discontinuers [25]. Caliper matching was defined as a caliper equal to 0.05 of the logit of the propensity score.
All statistical analyzes were performed using SAS 9.2 (or later versions) statistical software (SAS Institute Inc., Cary, NC, USA).
Results
Patient Demographics and Baseline Characteristics
Of the 5932 patients initiating liraglutide, 3580 fulfilled the inclusion criteria; of these, 2695 were classed as continuers and 885 as discontinuers (Fig. 1; Table 1). Of note, 76 (2.7%) continuers were excluded due to bariatric surgery compared with 85 (8.8%) discontinuers before matching.
Altogether, 882 patients in each group were matched 1:1 (Table 1). Mean ±SD propensity scores before matching were as follows: continuers 0.76 ± 0.05 (n = 2695) vs discontinuers 0.74 ± 0.06 (n = 885); after matching, the scores were: continuers 0.74 ± 0.06 (n = 882) vs discontinuers 0.74 ± 0.06 (n = 882). Apart from three cases, a matched case with a caliper of < 0.05 was found for each discontinuer.
Before matching, there were between-group differences in BMI and in the percentage of patients with existing comorbidities, such as CKD and pancreatitis. The percentages of patients taking insulin, metformin, sulfonylurea, DPP-4i, and acarbose, as well as the overall number of oGLDs also differed. After matching, there were between-group differences in the percentage of patients with CKD and in those taking metformin (Table 1).
Matched patients were ~ 60 years old, 44.0% were women, and the baseline HbA1c was 9.0% in both groups (Table 1). Among the continuers, 79.5% continued treatment for > 24 months, whereas half (50.8%) of discontinuers stopped treatment after ≤ 3 months, with only 11.2% completing 9 to < 12 months. Among the 882 continuers, 6.5% were treated for 12-15 months, 5.2% for 15–18 months, 3.5% for 18–21 months, 5.3% for 21–24 months, and 79.5% for > 24 months. Among the 882 discontinuers, 50.8%, 21.2%, 16.8%, and 11.2% were treated for ≤ 3, 3–6, 6–9, and 9 to < 12 months, respectively. Among all 3580 patients who met the inclusion criteria, 2191 (61.2%) persisted with the liraglutide treatment for more than 2 years.
Association Between Liraglutide Persistence and HbA1c Level
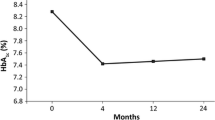
The change in HbA1c from baseline to 24 months between matched study groups was significantly greater in continuers than in discontinuers (p < 0.001). Mean reductions in HbA1c levels from baseline to 24 months in continuers was – 0.8% vs – 0.3% in discontinuers, both in matched and unmatched populations (Table 2).
A subanalysis stratified patients by HbA1c at baseline based on whether they were higher/lower than the median HbA1c (8.6%). This subanalysis showed that continuers with HbA1c > 8.6 at baseline had a greater mean reduction in HbA1c than discontinuers (– 1.32% vs – 0.71%, respectively; p < 0.001), despite similar baseline levels. Continuers with HbA1c ≤ 8.6 at baseline also had a greater reduction in HbA1c than discontinuers (– 0.29% vs 0.08%, respectively; p < 0.001).
Among patients who adhered to liraglutide for > 24 months and their matched discontinuers, the reduction in HbA1c from baseline to 24 months was also greater (– 0.8% vs – 0.27%, respectively; p < 0.001) for patients who used insulin at baseline (– 0.82% vs – 0.2%, respectively; p < 0.001) and for patients who used insulin at 21–24 months after the index date (– 0.77% vs – 0.28%, respectively; p < 0.001).
Association Between Liraglutide Persistence and Body Weight, BMI, and Blood Lipids
For both continuers and discontinuers, changes from baseline to 24 months in key measures were similar in the unmatched and matched groups (Table 2). Before matching, a total of 3.5% patients had missing values of change from baseline in body weight, 2.8% had missing values of change from baseline in LDL, and 2.4% had missing values of change from baseline in triglyceride. In the matched groups, mean body weight reductions were greater in continuers than in discontinuers (– 3.57 vs – 1.25 kg, respectively; p < 0.001) and for BMI (– 1.29 vs – 0.45 kg/m2, respectively; p < 0.001) (Table 2). For patients using insulin at 21–24 months, mean change in body weight from index date to 24 months significantly differed between continuers and discontinuers (– 3.22 vs – 0.11 kg, respectively; p < 0.001). No difference was observed between the groups for the change in blood lipids from baseline to 24 months (Table 2).
Association Between Liraglutide Persistence and Use of Insulin and Other Treatments
For both continuers and discontinuers, results were similar in the unmatched and matched groups (Table 3). In the matched groups, fewer continuers than discontinuers were dispensed insulin at 21–24 months after the index date (59.0% vs 70.2%, respectively; p < 0.001). Most continuers and discontinuers were taking one or two oGLDs at 21–24 months after the index date; however, the number of oGLDs dispensed was fewer for continuers than for discontinuers (p < 0.001). Of the oGLDs dispensed at 21–24 months after the index date, differences were seen between continuers and discontinuers for metformin (83.0% vs 77.2%; p = 0.002) and DPP-4i (p < 0.001). Fewer continuers than discontinuers used ≥ 3 oGLDs at 24 months following baseline (6.2% vs 20.6%, respectively; p < 0.001) (Table 3).
Association Between Liraglutide Persistence and Hospitalization
For both continuers and discontinuers, hospitalization results were similar in the unmatched and matched groups (Table 3). For the matched groups, fewer continuers were hospitalized during the 12–24 months post baseline than discontinuers (18.6% vs 22.8%, respectively; p = 0.034). Fewer, but not statistically significantly fewer, continuers than discontinuers were hospitalized for diabetes-related complications (6.5% vs 8.6%, respectively; p = 0.104) (Table 3). Similarly, among 701 patients who continued treatment for more than 24 months and their matched discontinuers, 15.8% of the continuers were hospitalized at 12–24 months compared with 23.4% of their matched discontinuers (p < 0.001); < 10.0% of each group were hospitalized for diabetes-related complications (5.1% for continuers vs 9.0% for discontinuers; p = 0.005).
Discussion
We found that patients with type 2 diabetes who persist with liraglutide treatment are characterized by a greater reduction in HbA1c, together with greater reductions in body weight and no changes to blood lipid levels, compared with patients who discontinued liraglutide therapy. Reductions in HbA1c after 24 months were greater in continuers than discontinuers in subgroups characterized by higher baseline HbA1c and by insulin usage at 21–24 months. Overall, persistence with liraglutide treatment was high, with ~ 60.0% of patients who initiated liraglutide persisting with the treatment for more than 2 years.
These results are supported by another real-world MHS one-arm study of patients with type 2 diabetes treated with liraglutide for ≥ 6 months [20], where liraglutide treatment resulted in a similar reduction in HbA1c and body weight to that observed in our study. In a separate retrospective cohort study of administrative claims data, HbA1c targets were more likely to be achieved by persistent than nonpersistent patients [26], both in unadjusted analysis and when adjusted to baseline covariates.
In our study, HbA1c reductions were greater in continuers than discontinuers, even though discontinuers were using more insulin and other oGLDs at follow-up measurement. In other studies that evaluated medication adherence and persistence, patient cohorts that also prescribed insulin or oGLDs at baseline showed between-group differences in medications. In one previous study, 60.0% of patients who had adhered to liraglutide at 1 year had a significantly higher mean number of oGLD medications at baseline [26]. In addition, previous use of medication has been shown to affect continuation with a subsequent medication [27], which may result from acquired tolerance to drugs within the same therapeutic class. In general, adherent patients will be more likely to achieve HbA1c goals.
In this study, continuers tended to remain on liraglutide treatment for a long time, while discontinuers mostly stopped therapy within the first 3 months. A previous liraglutide plus basal insulin study found that patients who discontinued therapy did so early, with 22.4% discontinuing in the first month and 39.5% within the first 3 months [28]. Adverse gastrointestinal events are more common in the early stages of treatment with GLP-1RAs, especially during the first 8 weeks [28]. Tolerability as a reason for nonadherence has previously been noted in a review examining factors that influence adherence to therapies for type 2 diabetes [29]. However, we did not report reasons for early treatment discontinuation.
Clinical outcomes likely play a role in treatment continuation/discontinuation as well. Since better persistence is associated with improvements in HbA1c outcomes, this positive effect could have influenced patients to remain on treatment. Nevertheless, continued use of GLP-1RAs may be compromised by adverse effects, particularly gastrointestinal events in the early treatment stages [30]. One study that sought to identify the main reasons why patients discontinue GLP-1RAs found that both patients and physicians reported gastrointestinal adverse events as a key reason, particularly nausea and vomiting. Physicians also considered poor blood glucose control as a key reason, and patients were particularly concerned with weight gain [31]. We believe that, since most patients discontinued within 3 months, side effects were likely to be the primary reason. However, in our study, some patients probably discontinued liraglutide due to lack of weight loss or lack of effect on HbA1c, since medications associated with reduced weight loss or fewer hypoglycemic events have been reported to increase patient satisfaction and adherence [29]. The cost of liraglutide treatment is not believed to be a key driver of discontinuation.
Our study demonstrated a positive association between adherence to liraglutide treatment and lower probability of needing bariatric surgery. More discontinuers than continuers were excluded from the study because they had undergone bariatric surgery during the study period (Fig. 1).
We noted that patients who persisted with their treatment were hospitalized less frequently than those discontinuing medication. The proportion of liraglutide-treated patients requiring an inpatient hospital stay has been reported to be low by other investigators [27, 32], and the odds of all-cause hospitalization are reportedly reduced by treatment adherence [32]. In another study, patients who persisted with a GLP-1RA and insulin also had a significantly lower number of hospitalizations and shorter hospital stays than nonpersistent patients [28]. Lower CV morbidity and mortality have been documented with liraglutide use on top of standard care in a previous trial, which could potentially lead to differences in hospitalizations between the two groups [10]. Prescription costs for the persistent cohort were, however, significantly higher [28]. In addition, persistence with liraglutide has been shown to be associated with significantly lower diabetes-related medical costs (includes costs relating to ambulatory visits and emergency/inpatient services) than for nonpersistent patients. However, total healthcare costs were higher for patients who persisted with treatment, due to higher diabetes-related pharmacy costs [26, 32].
This study has several strengths: the MHS has been a well-established care provider for over 75 years, and is the second largest Health Maintenance Organization in Israel, so data from it accurately reflect the Israeli population; it offers high-quality data from electronic medical records, automatic data capture, and a central laboratory; the considerable number of members enabled the inclusion of a large study population both overall and in matched groups. Because data were collected retrospectively from routine clinical practice, we had no influence on treatment decisions; the selection of liraglutide thus reflects real-life treatment decisions. However, as data were not collected from study-related clinical or laboratory monitoring or tests, clinical events may have been missed or only partly followed up, and the reason for discontinuation was not recorded.
In randomized controlled trials, randomization ensures that, in general, patient groups do not differ in their characteristics. In this study, we adjusted for differences in baseline characteristics by propensity score matching. However, residual confounding cannot be excluded, as suggested by differences in some characteristics after matching. No adjustments have been made for differences between continuers and discontinuers occurring after treatment initiation (e.g., side effects/lack of effect), which may have affected both long-term persistence and clinical outcomes. Our study design therefore precludes any conclusion on cause and effect of treatment continuation or discontinuation: poor control may have led to discontinuation of liraglutide, rather than vice versa. Another possible limitation is that the early response values (HbA1c) were measured 90–270 days after baseline, with the measurement closest to 180 days being used. This may be considered a particularly long period with which to measure the glycemic control before the change in treatment.
Other limitations should also be noted. A relatively large proportion of eligible individuals initiating liraglutide were excluded due to various database and/or study inclusion/exclusion criteria. This may limit the internal validity and generalizability of the study results. The high proportion of discontinuers who discontinued within the first 3 months may indicate that these patients were more likely to discontinue due to tolerability issues rather than a lack of effect on HbA1c. Suboptimal persistence with liraglutide in real-world settings (less than 30.0% after 2 years) has been shown in a previous study [33]. The influence of medications other than hypoglycemic agents on the study findings cannot be ruled out. Poor adherence to medications for comorbidities, including antihypertensive agents and lipid-lowering therapies, has also been shown to be an issue among patients with diabetes [29].
Conclusions
In a real-world setting of patients well treated for type 2 diabetes, persistent use of liraglutide was associated with good glycemic and body weight control. Adherent patients were also less likely to be hospitalized. Efforts should be made to increase persistence with therapy among new users.
References
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. https://www.aace.com/sites/all/files/diabetes-algorithm-executive-summary.pdf. Accessed 19 Jan 2018.
Jespersen MJ, Knop FK, Christensen M. GLP-1 agonists for type 2 diabetes: pharmacokinetic and toxicological considerations. Expert Opin Drug Metab Toxicol. 2013;9(1):17–29.
Neumiller JJ. Differential chemistry (structure), mechanism of action, and pharmacology of GLP-1 receptor agonists and DPP-4 inhibitors. J Am Pharm Assoc. 2003;2009(49 Suppl 1):S16–29.
Monami M, Zannoni S, Pala L, et al. Effects of glucagon-like peptide-1 receptor agonists on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Int J Cardiol. 2017;240:414–21.
Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39–47.
Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–81.
Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26(3):268–78.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care. 2009;32(1):84–90.
Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–55.
Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32(7):1224–30.
McAdam-Marx C, Nguyen H, Schauerhamer MB, et al. Glycemic control and weight outcomes for exenatide once weekly versus liraglutide in patients with type 2 diabetes: a 1-year retrospective cohort analysis. Clin Ther. 2016;38(12):2642–51.
Ostawal A, Mocevic E, Kragh N, et al. Clinical effectiveness of liraglutide in type 2 diabetes treatment in the real-world setting: a systematic literature review. Diabetes Ther. 2016;7(3):411–38.
Divino V, DeKoven M, Khan FA, et al. GLP-1 RA treatment patterns among type 2 diabetes patients in five European countries. Diabetes Ther. 2017;8(1):115–28.
Martinez L, Penfornis A, Gautier JF, et al. Effectiveness and persistence of liraglutide treatment among patients with type 2 diabetes treated in primary care and specialist settings: a subgroup analysis from the EVIDENCE study, a prospective, 2-year follow-up, observational, post-marketing study. Adv Ther. 2017;34(3):674–85.
Heymann AD, Chodick G, Halkin H, et al. The implementation of managed care for diabetes using medical informatics in a large preferred provider organization. Diabetes Res Clin Pract. 2006;71(3):290–8.
Heymann A, Maor Y, Goldstein I, et al. Efficacy of liraglutide in a real-life cohort. Diabetes Ther. 2014;5(1):193–206.
Chodick G, Heymann AD, Shalev V, et al. The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol. 2003;18(12):1143–6.
Shalev V, Chodick G, Goren I, et al. The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int J Cardiol. 2011;152(3):345–9.
Weitzman D, Chodick G, Shalev V, et al. Prevalence and factors associated with resistant hypertension in a large health maintenance organization in Israel. Hypertension. 2014;64(3):501–7.
Sawilowsky S. New effect size rules of thumb. J Modern Appl Stat Methods. 2009;8(2):597–9.
Parsons LS. Performing a 1:N case-control match on propensity score (paper 165-29). In: Proceedings of SUGI 29; 2004 May 9–12; Montreal, Canada. https://www2.sas.com/proceedings/sugi29/165-29.pdf. Accessed 22 Jan 2018.
Buysman EK, Liu F, Hammer M, et al. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32(4):341–55.
Yu M, Xie J, Fernandez Lando L, et al. Liraglutide versus exenatide once weekly: persistence, adherence, and early discontinuation. Clin Ther. 2016;38(1):149–60.
Lin J, Lingohr-Smith M, Fan T. Real-world medication persistence and outcomes associated with basal insulin and glucagon-like peptide 1 receptor agonist free-dose combination therapy in patients with type 2 diabetes in the US. Clinicoecon Outcomes Res. 2017;9:19–29.
Garcia-Perez LE, Alvarez M, Dilla T, et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–94.
Wilke T, Mueller S, Groth A, et al. Non-persistence and non-adherence of patients with type 2 diabetes mellitus in therapy with GLP-1 receptor agonists: a retrospective analysis. Diabetes Ther. 2016;7(1):105–24.
Sikirica MV, Martin AA, Wood R, et al. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403–12.
Nguyen H, Dufour R, Caldwell-Tarr A. Glucagon-like peptide-1 receptor agonist (GLP-1RA) therapy adherence for patients with type 2 diabetes in a medicare population. Adv Ther. 2017;34(3):658–73.
Gautier JF, Martinez L, Penfornis A, et al. Effectiveness and persistence with liraglutide among patients with type 2 diabetes in routine clinical practice—EVIDENCE: a prospective, 2-year follow-up, observational, post-marketing study. Adv Ther. 2015;32(9):838–53.
Acknowledgements
The authors would like to thank the participants of the study.
Funding
This study and article processing charges were funded by Novo Nordisk Health Care AG. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Editorial Assistance
Editorial assistance in the preparation of this manuscript was provided by Emma Fulkes, PhD, of PAREXEL, and was funded by Novo Nordisk Health Care AG.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Authorship Contributions
Cheli Melzer-Cohen performed the statistical analyses, with input from Lise Lotte N. Husemoen, Gabriel Chodick, and Varda Shalev. Nicolai Rhee and Avraham Karasik were involved in the study design. All authors interpreted the results, were involved with the writing of the manuscript, and approved the final draft.
Prior Presentation
This study was published as an abstract at the American Diabetes Association’s 78th Scientific Sessions, June 22–26, 2018, Orlando, Florida, USA; abstract A-2597.
Disclosures
Nicolai Rhee is an employee of Novo Nordisk. Lise Lotte N. Husemoen is an employee of Novo Nordisk. Avraham Karasik has received research grants and consulting fees from Novo Nordisk. The remaining authors Cheli Melzer-Cohen, Gabriel Chodick, and Varda Shalev have no conflict of interest to report.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were approved by the local institutional review board of Bayit Balev Rehabilitation Hospital, Israel, and were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Individual patient-informed consent was not required because of the anonymized nature of the patient records.
Data Availability
The data sets analyzed during the current study are not publicly available due to the ethics committee approval process for database studies in Israel, under which only specific researchers are permitted to access the data. Novo Nordisk’s policy on data sharing may be found at https://novonordisk-ctts.app-trialscope.com/how-access-clinical-trial-datasets.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7649636.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Melzer-Cohen, C., Chodick, G., Husemoen, L.L.N. et al. A Retrospective Database Study of Liraglutide Persistence Associated with Glycemic and Body Weight Control in Patients with Type 2 Diabetes. Diabetes Ther 10, 683–696 (2019). https://doi.org/10.1007/s13300-019-0583-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-0583-9