Abstract
Introduction
The goal of the study was to determine the level of metabolic compensation expressed by glycosylated hemoglobin, fasting plasma glucose, and postprandial glucose as determined after a standardized breakfast; further, to evaluate interrelationships between the studied parameters and postprandial glucose levels.
Methods
The study included 1055 patients with type 2 diabetes mellitus. Their fasting plasma glucose and postprandial glucose were measured before and after a standardized breakfast. Attending diabetologists completed a uniform questionnaire that included demographic data, type of antidiabetic treatment, duration of diabetes, latest glycosylated hemoglobin value, presence of dyslipidemia, and organic complications.
Results
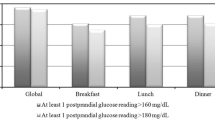
Glycosylated hemoglobin < 53 mmol/mol was achieved in 363 (34.2%), postprandial glucose < 7.5 mmol/l in 211 (19.9%), and fasting plasma glucose < 6 mmol/l in 251 (23.7%) patients. Excellent metabolic compensation, indicated by all the above mentioned glycosylated hemoglobin, fasting plasma glucose, and postprandial glucose values simultaneously, was achieved in only 71 (6.7%) patients. Comparable to fasting plasma glucose and postprandial glucose values, correlation with glycosylated hemoglobin levels is statistically significant; however, there is no difference at different glycosylated hemoglobin levels. There was a significant correlation between dyslipidemia and postprandial glycemia (p = 0.013).
Conclusion
The objective of care for patients with diabetes mellitus is to improve their long-term metabolic compensation; to that end, both fasting plasma glucose and postprandial glucose deserve equal attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DM (diabetes mellitus) is a progressive disease which over the years leads to metabolic complications [1, 2]. Formerly, the risk of these complications has been associated only with the level of glycosylated hemoglobin (HbA1c) which reflects long-term changes in glucose metabolism. At present, there are data available that indicate a close relationship between the development of late complications and postprandial glycemia (PPG), which is an independent risk factor of organic complications [3,4,5,6]. Taken together, PPG, fasting plasma glucose (FPG), and HbA1c then serve to assess metabolic compensation in the wider sense, forming the so-called glucose triad, all components of which should be addressed by treatment [7, 8]. Target values of PPG, FPG, and HbA1c should be individualized, particularly with regard to the choice of antidiabetic medication (risk of hypoglycemia), DM duration, life expectancy, presence of concurrent disorders, and complications [9].
Postprandial Plasma Glucose
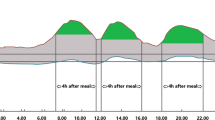
Postprandial plasma glucose levels are a direct measure of glucose concentrations in the blood following a meal, standardized generally at 2 h after eating (2 h PPG). In healthy individuals, glucose levels reach a peak approximately 1 h after ingestion of food and then return to premeal levels within 2–3 h [10]. Normal 2 h PPG levels are usually < 6.6 mmol/l and should not be > 7.8 mmol/l [11]. Such targets are individualized particularly with regard to the age of each patient and associated organic complications.
Postprandial hyperglycemia is a frequent occurrence in patients with type 2 diabetes, even at normal HbA1c levels, when PPG may become elevated. In a number of studies with type 2 DM patients that recorded glycemic profiles including PPG, elevation of PPG up to 8.9 mmol/l has been noted, despite HbA1c below 54 mmol/mol [12, 13].
PPG and its Relationship to Cardiovascular Disease
The relationship between hyperglycemia and cardiovascular disease is complex, with evidence suggesting that an acute increase of glycemia, particularly after a meal, may have a direct detrimental effect on cardiovascular disease [14,15,16,17]. The value of PPG monitoring has been demonstrated by analysis of the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) study population. The DECODE study group reported that 2 h PPG levels are a better predictor of on-study death from all causes and from cardiovascular disease than FPG levels [3].
Evidence from more recent studies notes that a marker of atherosclerosis corresponds better with the peak magnitude of patients’ postprandial glucose excursions than with both FPG and HbA1c levels [18, 19]. Specifically, it is thanks to knowing the FPG and PPG values that we are able to determine the extent of the variability which is the risk factor of organ damage. This fact was demonstrated also with relatively new technologies, such as the sensors of continuous glucose monitoring [20]. A study by Buscemi et al. [20] suggests that glycemic variability influences endothelial function even in non-diabetic subjects. Such variability may explain the increased cardiovascular risk observed in patients prior to developing overt type 2 DM. The negative impact of glycemic variability on vascular endothelium function can be explained by many factors, mainly by hyperglycemic memory with activation of oxidative stress [21], even in healthy individuals [22].
Primary Objective
The goal of epidemiological analysis of diabetic outpatients in the Czech Republic was to determine the level of metabolic compensation expressed by HbA1c, FPG, and PPG, and to determine the percentage of patients meeting the excellent metabolic compensation parameters according to standards of the Czech Diabetes Society.
Secondary Objective
To investigate the relationships between the collected demographic factors (gender, age, BMI, dyslipidemia, persistence length of diabetes, presence of late complications of diabetes, type of antidiabetic treatment, HbA1c, FPG) and measured level of PPG.
To determine the contribution of the FPG and PPG to different levels of HbA1c.
To evaluate the relevance of measured PPG levels for potential treatment change in subpopulations of diabetic patients.
Methods
This was an observational multicenter study with participation of physicians from diabetes outpatient departments in the Czech Republic. In total, the study involved 1055 subjects with type 2 DM. Recruited outpatients came from consulting rooms of general diabetologists and were included as they attended ordinary visits to their doctor. The only inclusion criteria for the study were type 2 DM, age > 18 years, and signed informed consent. The level of HbA1c (values are given in millimoles per mole according to the IFCC calibration method) was not an exclusion/inclusion criterion in the study. The patients’ FPG and PPG were measured before and after a standardized breakfast—a ham baguette Crocodile (contains 268.4 kcal, 11.52 g protein, 22.70 g carbohydrates, 16.56 g lipids). Attending diabetologists completed a uniform questionnaire that included demographic data, type of antidiabetic treatment, DM duration, latest know HbA1c value, presence of dyslipidemia and organic complications, and finally a response to a query concerning the significance of PPG for further treatment. Metabolic compensation target values were assessed by current care standards of the Czech Diabetes Society (http://www.diab.cz) [23].
The relationships between compensation indicators were evaluated by 2-factor ANOVA for interaction between the factors. The ANOVA was followed by multiple comparison tests (least significant difference test). Simultaneous evaluation of the relationships between indicators of diabetes compensation and the studied parameters was carried out by multiple regression with dimension reduction. Dichotomic data dependencies were tested by Fisher’s exact test. Levels of PPG in subjects with dyslipidemia vs subjects with normal serum lipids were evaluated using robust Mann–Whitney tests.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 (as revised in 2013). Informed consent was obtained from all patients to be included in the study.
Results
In total, 1055 subjects with type 2 DM participated in the study. The characteristics of the patients are presented in Table 1.
Within the framework of metabolic compensation evaluation, HbA1c < 53 mmol/mol was achieved in 363 (34.2%), PPG < 7.5 mmol/l in 211 (19.9%), and FPG < 6 mmol/l in 251 (23.7%) patients. Excellent metabolic compensation, indicated by all the above HbA1c, FPG, and PPG values simultaneously, occurred in only 71 (6.7%) patients.
In our study PPG correlated with HbA1c levels comparably with FPG. The correlation for FPG was r = 0.472 (p < 0.001) and for PPG it was r = 0.491 (p < 0.001). The correlation for PPG vs FPG did not statistically differ. The correlation of PPG of the group on oral antidiabetic agents (OADs) only vs those on insulin with/without OADs was r = 0.433 (p < 0.001) vs r = 0.371 (p < 0.001) and for FPG it was r = 0.384 (p < 0.001) vs r = 0.388 (p < 0.001). The impact of antidiabetic medication on the level of PPG was not found.
In the studied group the PPG contribution did not differ at different HbA1c levels.
Patients with dyslipidemia had increased PPG levels vs patients with normal lipid control—9.4 mmol/l (7.8; 12.0) vs 10.1 mmol/l (8.0; 12.8) [median (lower; upper limit)]. The difference was statistically significant (p = 0.013).
Microvascular complications were present in 350 (33.2%) and macrovascular complications in 355 (33.6%) patients.
According to the responding doctors, PPG was relevant for change in treatment in 807 cases (76.5%).
Antidiabetic treatment of study subjects is presented in Table 2.
Discussion
The study was designed to determine the level of metabolic compensation, particularly the level of PPG in the diabetic outpatient population in the Czech Republic. Its aim was not to test individual treatment regimens but rather the parameters of glucose metabolism compensation and under different treatments. HbA1c < 53 mmol/mol was achieved in 363 (34.2%), PPG < 7.5 mmol/l in 211 (19.9%), and FPG < 6 mmol/l in 251 (23.7%) patients. Excellent metabolic compensation, indicated by achieving these HbA1c, PPG, and FPG values simultaneously, as recommended by the Czech Diabetes Society, was achieved in only 71 (6.7%) patients.
A discrepancy in the percentage representation of individual parameters of the target metabolic compensation must be pointed out. If only 6.7% of the patients meet all three parameters, we can estimate that, for example, a PPG < 7.5 mmol/l will not bring about a target HbA1c < 53 mmol/mol, in which case the desired PPG value could be higher, such as < 10.0 mmol/l.
PPG measurement is still often neglected, particularly in type 2 DM patients, who do not receive insulin treatment and in whom frequently only FPG is being determined. And yet, a DM patient is in a postprandial state for most of the time during the day and PPG therefore can markedly affect the resulting HbA1c. Recent studies have focused on determining its PPG contribution to overall HbA1c levels. Reports indicate that postprandial hyperglycemia contributes approximately 70% of the total glycemic burden at HbA1c levels < 56 mmol/mol, decreasing to around 30% at HbA1c levels > 89 mmol/mol. The contribution of PPG to the resulting HbA1c is greater, the lower the HbA1c is [24, 25]. In contrast, the contribution of FPG increases with increasing HbA1c levels, suggesting that PPG may be a better indicator of glycemic control than FPG in patients with moderately elevated blood glucose [26]. Support for this hypothesis is provided by data suggesting that treatment aimed at reducing postprandial glucose excursions is more effective in lowering HbA1c levels than FPG-targeted therapy [27]. A number of randomized controlled trials have shown that patients treated with twice daily biphasic insulin, incorporating a rapid-acting analogue, achieved significantly lower HbA1c levels, compared with patients receiving a long-acting basal insulin [28,29,30]. Antidiabetic medication preferentially targeting PPG levels can bring other benefits as well, such as alleviation of endothelial dysfunction. Regimens using rapid-acting insulin analogues are effective both in reducing arterial oxidative stress and in improving endothelial dysfunction [31, 32]. OADs from the α-glucosidase inhibitors (AGIs), glinide classes, and gliptins have also been shown to improve markers of atherosclerosis in patients with type 2 diabetes [33,34,35]. Indeed, the benefits of the AGI acarbose translate into significant reduction in the risk of cardiovascular disease in patients with prediabetes, impaired glucose tolerance [36]. As the PPG is an independent risk factor of vessel wall damage it should be considered in the comprehensive management plan of individuals with diabetes. This should be taken into account when choosing antidiabetic medication, which should primarily target PPG [27].
We tested the impact of antidiabetic medication of participating subjects on their levels of PPG. This hypothesis was not borne out by our study. None of the administrated medications had any favorable impact on PPG levels. The group treated only with OADs did not differ in the impact on PPG compared to insulin (with/without OADs) treated patients.
In our study we investigated the contribution of PPG and FPG to overall HbA1c levels. There are insufficient data to determine accurately the relative contribution of the FPG and PPG to HbA1c It appears that FPG is somewhat better than PPG in predicting HbA1c, especially in type 2 diabetes [10]. In our study PPG correlated with HbA1c levels comparably with FPG. In the studied group, the PPG contribution did not differ at various HbA1c levels. This can be explained by the fact that the range of studied HbA1c values were below threshold for suboptimal control, especially in those patients on OADs. (The level of HbA1c was not an exclusion/inclusion criterion in the study.) We also did not find a different contribution of the PPG level in patients treated with insulinotherapy where the HbA1c was higher [for OADs 55.55 mmol/mol (55.11; 55.99) vs insulinotherapy 71.31 mmol/mol (70.31; 72.32)].
In our study we found increased PPG level in patients with dyslipidemia vs patients with normal lipid control (p = 0.013). Hyperlipidemia and hyperglycemia together represent a malignant combination for a risk of vascular complication. It is generally understood that dyslipidemia is closely related to metabolic compensation mainly in case of type 1 DM patients, whilst in case of patients with type 2 DM the lipid profile is more likely to be a factor of insulin resistance and metabolic syndrome. On the other hand our study showed that PPG correlates with dyslipidemia also in the case of type 2 DM patients. Chronic elevations of glucose and/or lipids might damage β-cells, eventually enhancing pre-existing insulin resistance and insulin deficiency (glucolipotoxicity). Both abnormalities should be therefore addressed in the treatment strategy.
In our study only 55.4% of patients were treated with metformin. Even if metformin treatment contraindications (renal, respiratory, or cardiac insufficiency) are taken into account, the frequency of its administration can be considered deficient with regard to guidelines for type 2 DM treatment [9].
Conclusions
The objective of care for DM patients is to improve their long-term metabolic compensation; to that end, FPG and PPG deserve equal attention, as both represent measures essential for prevention of cardiovascular disorders in diabetics.
References
Caprnda M, Mesarosova D, Fabuel OP, et al. Glycemic variability and vascular complications in patients with type 2 diabetes mellitus. Folia Med. 2017;59(3):270–8.
Lin CC, Li CI, Yang SY, et al. Variation of fasting plasma glucose: a predictor of mortality in patients with type 2 diabetes. Am J Med. 2012;125(4):416.e9–18. https://doi.org/10.1016/j.amjmed.2011.07.027.
DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161(3):397–405.
Xu F, Zhao LH, Su JB, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6(1):139. https://doi.org/10.1186/1758-5996-6-139 (eCollection 2014).
Su G, Mi SH, Tao H, et al. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 2013;36(4):1026–32.
Esposito K, Giugliano D, Nappo F, Marfella R. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–9.
Monnier L, Colette C. Targeting prandial hyperglycemia: how important is it and how best to do this? Curr Diab Rep. 2008;8(5):368–74.
Monnier L, Colette C. Postprandial and basal hyperglycemia in type 2 diabetes: contributions to overall glucose exposure and diabetic complications. Diabetes Metab. 2015;41(6 Suppl 1):6S9–15. https://doi.org/10.1016/s1262-3636(16)30003-9.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–42. https://doi.org/10.1007/s00125-014-3460-0.
American Diabetes Association. Postprandial blood glucose. Diabetes Care. 2001;24:775–8.
Ceriello A, Colagiuri S, Gerich J, Tuomilehto J, Guideline Development Group. Guideline for management of postmeal glucose. Nutr Metab Cardiovasc Dis. 2008;18(4):S17–33. https://doi.org/10.1016/j.numecd.2008.01.012.
Bonora E, Calcaterra F, Lombardi S, et al. Plasma glucose levels through-out the day and HbA1c interrelationships in type 2 diabetes: implications for treatment and monitoring of metabolic control. Diabetes Care. 2001;24:2023–9.
Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise degradation of fasting with worsening diabetes. Diabetes Care. 2007;30:263–9.
Ceriello A, Hanefeld M, Leiter L, et al. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164(19):2090–5.
Hanefeld M, Cagatay M, Petrowistch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10–6.
Hanefeld M, Chiasson J-L, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschien T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004;36:1073–8.
NAVIGATOR Study Group. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463–76.
Esposito K, Ciotola M, Carleo D, et al. Post-meal glucose peaks at home associate with carotid intima-media thickness in type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2639–46. https://doi.org/10.1210/jc.2007-200.
Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813–9.
Buscemi S, Re A, Batsis JA, et al. Glycemic variability using continuous glucose monitoring and endothelial function in the metabolic syndrome and in type 2 diabetes. Diabet Med. 2010;27(8):872–8. https://doi.org/10.1111/j.1464-5491.2010.03059.
Gradinaru D, Borsa C, Ionescu C, Margina D. Advanced oxidative and glycoxidative protein damage markers in the elderly with type 2 diabetes. J Proteomics. 2013;30(92):313–22. https://doi.org/10.1016/j.jprot.2013.03.034.
Mah E, Bruno RS. Postprandial hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutr Res. 2012;32(10):727–40. https://doi.org/10.1016/j.nutres.2012.08.002.
Guidelines of Czech Society of Diabetes. http://www.diab.cz.
Monnier L, Colette C, Boniface H. Contribution of postprandial glucose to chronic hyperglycemia: from the “glucose triad” to the trilogy of “sevens”. Diabetes Metab. 2006;32(Spec No2):2S11–6.
Monnier L, Colette C, Owens D. Postprandial and basal glucose in type 2 diabetes: assessment and respective impacts. Diabetes Technol Ther. 2011;13(Suppl 1):S25–32. https://doi.org/10.1089/dia.2010.0239.
Woo V, Shestakova MV, Ørskov C, Ceriello A. Targets and tactics: the relative importance of HbA1c, fasting and postprandial plasma glucose levels to glycemic control in type 2 diabetes. Int J Clin Pract. 2008;62(12):1935–42. https://doi.org/10.1111/j.1742-1241.2008.01941.x.
Aronoff SL. Rationale for treatment options for mealtime glucose control in patient with type 2 diabetes. Postgrad Med. 2017;129(2):231–41. https://doi.org/10.1080/00325481.2017.1285191.
Raskin P, Allen E, Hollander P, et al. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–5.
Malone JK, Kerr LF, Campaigne BN, Sachson RA, Holcombe JH, Lispro Mixture-Glargine Study Group. Combined therapy with insulin lispro Mix 75/25 plus metformin or insulin glargine plus metformin: a 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapy. Clin Ther. 2004;26(12):2034–44.
Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycemic control in patients with type 2 diabetes. Diabet Med. 2005;22(4):374–81.
Ceriello A, Quagliaro L, Catone B, et al. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25(8):1439–43.
Ceriello A, Cavarape A, Martinelli L, et al. The post-prandial state in type 2 diabetes and endothelial dysfunction: effects of insulin aspart. Diabet Med. 2004;21(2):171–5.
Hanefeld M, Chiasson JL, Koehler C, et al. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004;35(5):1073–8.
Mita T, Watada H, Shimizu T, et al. Nateglinide reduces carotid intima-media thickening in type 2 diabetic patients under good glycemic control. Arterioscler Thromb Vasc Biol. 2007;27(11):2456–62.
Barbieri M, Rizzo MR, Marfella R, et al. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis. 2013;227(2):349–54. https://doi.org/10.1016/j.atherosclerosis.2012.12.018.
Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–94.
Acknowledgements
Funding
The project was initiated with support from the Czech Diabetes Society and Eli Lilly and Company.
Authorship
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Denisa Janíčková Žďárská, Martin Hill, Milan Kvapil, Pavlína Piťhová, and Jan Brož have nothing to disclose.
Compliance with Ethical Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
The datasets obtained and analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to https://doi.org/10.6084/m9.figshare.5873433.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Žďárská, D.J., Hill, M., Kvapil, M. et al. Analysis of Postprandial Glycemia in Relation to Metabolic Compensation and Other Observed Parameters of Outpatients with Type 2 Diabetes Mellitus in the Czech Republic. Diabetes Ther 9, 665–672 (2018). https://doi.org/10.1007/s13300-018-0379-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0379-3