Abstract
Introduction
Basal insulin should be injected at the same time each day, but people with diabetes sometimes mistime their injections. It is not known whether irregular daily dose timing affects diabetes-related factors. We report here our evaluation of the effects of deviations from a regular dosing schedule on glycemic control and hypoglycemia on patients treated with long-acting insulin (insulin glargine U100). We also consider the effects of ultra-long-acting insulin (insulin degludec) in this context.
Methods
Nineteen individuals with type 1 diabetes and 58 with type 2 diabetes were enrolled. Demographic data on all participants were retrieved from their medical records. Variation in dose timing was determined as the difference between the time of the earliest mistimed dose and the time of the latest mistimed dose, for each participant, over a 2-week period. All participants completed the Summary of Diabetes Self-Care Activities questionnaire, Problem Areas in Diabetes scale and 5-Item World Health Organization Well-being Index. Glargine U100 was switched to degludec in those individuals with type 2 diabetes who achieved inadequate glycemic control or suffered from frequent hypoglycemic episodes or who required two injections per day, and changes in hemoglobin A1c level and frequency of hypoglycemic episodes during the 12-week period were compared.
Results
A greater difference in dose timing was related to a higher frequency of hypoglycemic episodes and overweight in persons with type 2 diabetes. Smoking, drinking and living alone were independently associated with a greater difference in dose timing. Insulin degludec decreased the frequency of hypoglycemia and improved glycemic control in participants whose dose mistiming was >120 min.
Conclusion
Fixed dose timing should be employed for basal insulin, as a larger difference in dose timing worsens diabetes-related factors. Insulin degludec improved glycemic control and lowered the hypoglycemia rate in persons with more irregular dose timing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Basal insulin is frequently used to treat all types of diabetes mellitus. The position statement of the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia in persons with type 2 diabetes states that basal insulin should be considered an essential component of the treatment strategy for any persons not achieving an agreed-upon glycated hemoglobin (HbA1c) target [1]. Basal insulin is also indispensable for persons with type 1 diabetes to maintain glycemic control within the target range and participate in normal daily activities [2].
During the last decade, long-acting insulin (glargine U100 and detemir) has become the main basal insulin in both type 1 and type 2 diabetes, as it contributes to better glycemic control and fewer hypoglycemic episodes when compared to intermediate-acting insulins [3–5]. A recent study also reported that patient-led insulin glargine titration achieved near-target blood glucose levels in uncontrolled type 1 [6] and type 2 diabetes [7]. The treatment of most patients with type 1 and type 2 diabetes consists of injections of long-acting insulins once a day; these insulins should be injected at the same time each day because the effectiveness of each dose is close to 24 h [8–14].
However, it is sometimes difficult and inconvenient for people with diabetes to follow clinical practice guidelines and inject themselves with long-acting insulin at the same time each day. Indeed, it has been reported that 27.6% of people with type 1 or type 2 diabetes have difficulty maintaining a regular daily dose schedule (i.e. injecting themselves with insulin at the same time each day). Physicians have generally considered that 81.4% of people with diabetes fail to take basal insulin at the same time each day and that 71.1% fail to take basal insulin due to a busy lifestyle or travel commitments [15]. In general, most people with diabetes find it difficult to incorporate basal insulin injections into their daily life, partly due to changing daily routines [16].
It is as yet unknown whether differences in the injection time of long-acting insulin affects glycemic control and safety in the clinical setting. We therefore compared variations in injection dose timing of insulin glargine U100 in daily life in people with type 1 or type 2 diabetes in terms of its association with glycemic control, hypoglycemia (frequency of hypoglycemic episodes) and body weight. We also evaluated whether an ultra-long-acting insulin, insulin degludec, has a beneficial effect on glycemic control and hypoglycemia in persons with type 2 diabetes who were found to have large variations in dose timing, inadequate glycemic control or frequent hypoglycemic episodes.
We found that 78.9% of persons with type 1 diabetes self-administered insulin glargine U100 within 120 min of the agreed dosing time. In those with type 2 diabetes, however, approximately 50% reported irregularities in the timing of glargine U100 injections that were beyond 120 min of the agreed dosing time. We also found that a variation in dose timing of >240 min was associated with an increased frequency of hypoglycemia and that an even larger difference in dose timing induced weight gain. Switching the type 2 diabetes patient from insulin glargine U100 to insulin degludec improved glycemic control and decreased the frequency of hypoglycemia in participants who tended to mistime doses by ≥120 min in a 12-week investigation.
Methods
Participants
Outpatients of Kyoto University Hospital were recruited continuously for the study. The outpatients eligible for inclusion were those aged ≥20 years with type 1 diabetes treated with bolus insulin and long-acting insulin (glargine U100) or type 2 diabetes treated with long-acting insulin (glargine U100) ± bolus insulin ± oral antidiabetic agents. Exclusion criteria were outpatients who received insulin injection by someone other than themselves, had severe diabetes complications and comorbidities (e.g. severe cardiovascular disease, liver and renal disorders, anemia, malignancy, dementia, sarcopenia), secondary diabetes, depression or psychiatric problems, were unable to follow trial procedures and/or were judged unsuitable for this study by physicians. The study protocol was approved by the Institutional Review Board of Kyoto University Hospital (E2313). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all participants for inclusion in the study. A total of 23 persons with type 1 diabetes and 66 persons with type 2 diabetes were judged eligible for entry into the survey; ultimately 22 persons with type 1 diabetes (95.7%) and 63 persons with type 2 diabetes (95.5%) participated following refusal by some of those eligible. Excluding those with incomplete data, 19 type 1 diabetes (82.6%) and 58 type 2 diabetes (87.9%) outpatients were included in the final analysis.
Procedures and Measurements
This was a cross-sectional study. All data were collected by certified diabetes educators between July and December 2014. Participants were asked to record dose timing of glargine U100 for 2 weeks. Difference in dose timing was determined as the time difference (min) between the earliest and the latest injection time during a 2-week period, as 2 weeks of monitoring is believed to reflect glycemic control during the subsequent 3 months [17]. All participants completed questionnaires on self-management performance and psychological state. Self-management performance was evaluated by The Summary of Diabetes Self-Care Activities (SDSCA) questionnaire [18, 19], were a higher mean score for each subscale indicates a higher level of each self-care practice. Psychological states were measured using the Problem Areas in Diabetes (PAID) scale [20, 21] and the 5-Item World Health Organization Well-being Index (WHO-5) [22–24].
The demographics of all participants were retrieved from medical records and by self-reporting, including age, sex, academic background, family structure, work status and drinking and smoking habits. Hypoglycemia was confirmed by the self-monitoring record of blood glucose during a 1-month period, including the 2-week recording of injection times, and was defined as a blood glucose level of ≤70 mg/dl [2, 25]. Body mass index (BMI) was calculated as body weight (kilogram) divided by height (meter) squared.
We retrospectively evaluated glycemic control (HbA1c) and frequency of hypoglycemia (episodes/month) in persons with type 2 diabetes who met specific criteria during a 12-week period based on the difference in dose timing after the study participant had been switched from insulin glargine U100 to ultra-long-acting insulin (insulin degludec). For entry into this specific analysis, participants with type 2 diabetes had to meet the following criteria based on the results of the preceding 2-weeks: HbA1c >8.0%; more than one hypoglycemic episode per week; twice-daily injection of glargine U100; variation in injection time of >120 min from the scheduled dosing; forgetting to administer an insulin glargine U100 injection.
Statistical Analysis
Data are expressed as the mean ± standard deviation for continuous variables and as a number with percentage for categorical variables, unless otherwise noted. Student’s t test was used to compare continuous variables of participant demographics and difference in dose timing of insulin glargine U100, and the Chi-square test was used to compare categorized variables. To examine the relationship between variables, we used bivariate Pearson correlations and to compare the mean HbA1c level, fasting plasma glucose levels and frequency of hypoglycemia between groups separated by the difference in dose timing of insulin glargine U100, we used the Dunnett multiple comparison test. A multiple linear regression analysis using a stepwise variable entry method was performed in type 2 diabetes to evaluate the factors associated with dose timing of insulin glargine U100. The independent variables were selected based on hypothesis testing or associations of correlations relevant to the demographic data, participant background and lifestyle habits (a p value of <0.2 was set for inclusion). Age and sex were adjusted in the multiple linear regression analysis.
Finally, the dependent samples Student’s t test was used to compare the mean of HbA1c level and the frequency of hypoglycemia between 0 and 12 weeks after switching the type 2 diabetes patient from insulin glargine U100 to insulin degludec. For all analyses, p values of <0.05 were considered to be statistically significant. Data analyses were performed using SPSS statistics version 20.0 (IBM Corp., Armonk, NY).
Results
Participants
Of the 23 persons with type 1 diabetes and 66 persons with type 2 diabetes eligible for entry into the study, 19 (82.6%) of those with type 1 diabetes and 58 (87.9%) of those with type 2 diabetes completed the study (Table 1).
Study participants with type 1 diabetes (42.1% female) had a mean age of 55.8 ± 21.8 years, mean BMI of 22.2 ± 3.0 kg/m2, mean HbA1c level of 7.8 ± 1.0% (62.0 ± 10.9 mmol/mol), mean fasting plasma glucose level of 171.2 ± 89.9 mg/dl; the frequency of hypoglycemia was 7.7 ± 9.9 episodes/month. The total daily insulin dose was 34.4 ± 13.2 U, and the dose of insulin glargine U100 was 15.1 ± 8.7 U. The mean difference in dose timing of insulin glargine U100 was 101.2 ± 48.6 min. Of these persons with type 1 diabetes, 47.4% were administered insulin glargine U100 twice daily. There was no significant difference in baseline characteristics between those with once-daily and twice-daily injections (data not shown).
Study participants with type 2 diabetes (39.7% female) had a mean age of 68.9 ± 11.2 years, mean BMI of 24.5 ± 4.0 kg/m2, mean HbA1c level of 8.0 ± 1.1% (64.0 ± 12.0 mmol/mol), mean fasting plasma glucose level of 146.2 ± 41.0 mg/dl; the frequency of hypoglycemia was 1.3 ± 3.3 episodes/month. The total daily insulin dose was 24.3 ± 16.0 U, and the daily dose of insulin glargine U100 was 16.1 ± 8.8 U. The mean difference in dose timing of insulin glargine U100 was 128.2 ± 82.9 min. Of these persons with type 2 diabetes, 22.4% were administered insulin glargine U100 twice daily. There were no significant differences in HbA1c and frequency of hypoglycemia between those with once-daily and twice-daily injections, but the total daily dose of insulin glargine U100 was significantly higher for those receiving twice-daily injections (data not shown).
Bolus insulin, sulfonylureas, metformin and a dipeptidyl peptidase-4 inhibitor were used by 21 (36.2%), 26 (44.8%), 6 (10.3%), and 36 (62.1%) study participants, respectively. There were significant differences in age (p = 0.020), BMI (p = 0.031), frequency of hypoglycemia (p = 0.012) and total daily insulin dose (p = 0.015) between persons with type 1 diabetes and those with type 2 diabetes.
Difference in Dose Timing of Insulin Glargine U100 and Glycemic Control
There were dose timing differences for insulin glargine U100 among the study participants with type 1 diabetes, with 5 persons (26.3%) found to have a dose time difference of 0–60 min, 10 persons (52.6%) with a dose time difference of 61–120 min, 3 persons (15.8%) with a dose time difference of 121–180 min and 1 person (5.3%) with a dose time difference of 181–240 min. Corresponding to these increasing time differences, the mean HbA1c levels [± standard error (SE)] were 7.9 ± 0.5% (63.0 ± 5.5 mmol/mol), 7.6 ± 0.3% (60.0 ± 3.3 mmol/mol), 8.6 ± 0.6% (70.0 ± 6.6 mmol/mol) and 6.7% (50 mmol/mol), respectively (Fig. 1a); fasting plasma glucose levels were 154.0 ± 39.8, 180.5 ± 34.5, 171.0 ± 26.6 and 165.0 mg/dl, respectively (Fig. 1b); frequency of hypoglycemia was 12.0 ± 6.5 , 7.8 ± 2.7, 1.0 ± 1.0 and 5.0 episodes/month, respectively (Fig. 1c). There was no significant difference in HbA1c levels, fasting plasma glucose levels or frequency of hypoglycemia among these four levels of mistiming in patients with type 1 diabetes.
Dose timing differences for glargine U100 were also found among the study participants with type 2 diabetes, with 20 persons (34.5%) found to have a dose time difference of 0–60 min, 12 persons (20.7%) with a dose time difference of 61–120 min, 13 persons (22.4%) with a dose time difference of 121–180 min, 7 persons (12.1%) with a dose time difference of 181–240 min and 6 persons (10.3%) with a dose time difference of 241–300 min. Corresponding to these increasing time differences, the mean HbA1c levels (± SE) were 8.0 ± 0.2% (64.0 ± 2.2 mmol/mol), 8.0 ± 0.3% (64.0 ± 3.3 mmol/mol), 8.1 ± 0.4% (65.0 ± 4.4 mmol/mol), 8.0 ± 0.5% (64.0 ± 5.5 mmol/mol) and 8.1 ± 0.4% (65.0 ± 4.4 mmol/mol), respectively (Fig. 1d); the mean fasting plasma glucose levels were 141.5 ± 8.5, 148.4 ± 13.0, 136.8 ± 9.1, 144.6 ± 17.2 and 159.0 ± 13.7 mg/dl, respectively (Fig. 1e); the frequency of hypoglycemia was 0.7 ± 0.4, 0.9 ± 0.4, 1.2 ± 0.5, 1.1 ± 1.1 and 4.7 ± 3.5 episodes/month, respectively (Fig. 1f). HbA1c levels and fasting glucose levels were not significantly different among the five levels of mistiming. The frequency of hypoglycemia was higher in the 241–300 min group than in the 0–60 min group (p = 0.042).
Correlation Between Difference in Dose Timing of Insulin Glargine U100 and Age and Physical Findings
The age of persons with type 1 diabetes was negatively correlated with a larger difference in dose timing of insulin glargine U100 (r = −0.544, p = 0.016) (Fig. 2a); body weight (p = 0.869) and BMI (p = 0.965) were not correlated with dose timing (Fig. 2b, c).
The age of persons with type 2 diabetes was not correlated with dose timing (p = 0.379) (Fig. 2d); however, body weight (r = 0.322, p = 0.014) and BMI (r = 0.276, p = 0.036) were positively correlated with increased variation in dose timing of insulin glargine U100 (Fig. 2e, f).
Blood pressure, pulse rate, and diabetes complications were not correlated with variation in dose timing (data not shown).
Association Between Difference in Dose Timing of Glargine U100 and Study Participant Background, Insulin Therapy and Lifestyle
Among persons with type 1 diabetes, sex did not affect difference in dose timing (data not shown). Other background factors could not be analyzed due to the small number of enrolled participants.
Among persons with type 2 diabetes, there were no statistically significant differences in the correlations between variations in dose timing and sex, frequency of basal insulin injection (once daily vs. twice daily), insulin therapy (basal supported oral therapy vs. basal bolus therapy) or work status (Fig. 3a–f). However, the difference in dose timing of insulin glargine U100 was greater in more highly educated participants (p = 0.040) (postgraduate vs. junior high school graduate: 174.5 ± 63.7 vs. 47.6 ± 12.3 min, p = 0.017) (Fig. 3d). Similarly, living alone (194.0 ± 41.5 vs. 95.7 ± 12.7 min, p = 0.009) and drinking habits (149.0 ± 25.9 vs. 87.0 ± 13.2 min, p = 0.024) were associated with an increased variation in dose timing, respectively. (Figure 3e, g). Smokers also tended to have a greater variation in dose timing (Fig. 3h). In the multiple linear regression analysis, living alone [β = 125.5, 95% confidence interval (CI) 63.5–187.5, p < 0.001], drinking (β = 75.0, 95% CI 22.4–127.7, p = 0.007) and smoking (β = 99.0, 95% CI 27.8–170.2, p = 0.008) remained significantly associated with greater differences in dose timing.
Correlation between difference in dose timing of insulin glargine U100 and study participant’s background (a sex, b insulin therapy, c frequency of insulin glargine, d academic background, e family structure, f work status) and lifestyle habits (g drinking habits, h smoking habit) in persons with type 2 diabetes. BOT Basal-supported oral therapy, BBT basal bolus therapy, IGla insulin gargine U100. * Significantly different at p < 0.05
Self-Management Performance and Psychological States in Persons with Type 2 diabetes
The diet and exercise scores were not correlated with dose timing of insulin glargine U100, but they were negatively correlated with the medication score in SDSCA (r = −0.365, p = 0.029). PAID scale and WHO-5 index scores were not significantly correlated with increased differences in dose timing.
Effects of Switching From Insulin Glargine U100 to Insulin Degludec on Glycemic Control and Frequency of Hypoglycemia in Persons with Type 2 diabetes
The therapeutic regimen of 23 study participants with type 2 diabetes was switched from insulin glargine U100 to insulin degludec (Table 2). According to the median difference in dose timing of insulin glargine U100 (120.0 min), these persons were assigned to two groups: 12 in a group with a dose time difference of 0–120 min (0–120 min group) and 11 in a group with a dose time difference of 121–240 min (121–240 min group). There were no significant differences in participant demographics between the two groups. The mean difference in dose timing in the 0–120 min group was 60.1 ± 38.0 min and that in the 121–240 min group was 203.6 ± 34.1 min, which is a statistically significant difference (p < 0.001).
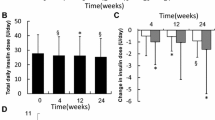
Of those persons with type 2 diabetes who were switched from insulin glargine U100 to insulin degludec, 39.1% were changed from insulin glargine U100 twice daily to insulin degludec once daily. To avoid hypoglycemia, the starting dose of insulin degludec was decreased by about 20% according to the Japanese label [26], from 21.4 ± 11.4 to 17.4 ± 7.8 U in the 0–120 min group (p = 0.009) and from 17.9 ± 8.6 to 15.5 ± 7.3 U in the 121–240 min group (p = 0.005) (Fig. 4a, b). During the12-week period, the doses were slightly increased and the final dose was 18.4 ± 9.0 U in the 0–120 min group; the doses reached baseline levels without significant difference in the 121–240 min group. The total dose of bolus insulin was not changed during the 12-week period in either group.
Insulin doses (a, b), HbA1c levels (c, d) and frequency of hypoglycemia (e, f) in study participants with a dose time difference of 0–120 min (0–120 min; a, c, e) and with a dose time difference of 121–240 min (121–240 min; b, d, f) at the initiation of the switch to insulin degludec from insulin glargine U100 (0 week after switching) and 12 weeks after the switch (12 weeks after switching). * Significantly different at p < 0.05
HbA1c levels decreased from 8.2 ± 1.2% (66.0 ± 13.1 mmol/mol) to 8.1 ± 1.0% (65.0 ± 10.9 mmol/mol) (−0.15%, 95% CI −0.22–0.52 [−1.6 mmol/mol, 95% CI −2.4–5.7]) in the 0–120 min group; this difference was not statistically significant (Fig. 4c). HbA1c levels decreased from 8.0 ± 1.2% (64.0 ± 13.1 mmol/mol) to 7.7 ± 1.3% (61.0 ± 14.2 mmol/mol) (−0.31%, 95% CI −0.02−0.60 [−3.4 mmol/mol, 95% CI −0.2−6.6]) in the 121–240 min group (Fig. 4d); this difference was statistically significant (p = 0.039).
The frequency of hypoglycemia did not change significantly in the 0–120 min group (from 0.2 ± 0.4 to 0.4 ± 0.8 episodes/month; −0.15, 95% CI −0.22–0.52) (Fig. 4e). However, the frequency of hypoglycemia was significantly decreased from 3.8 ± 7.9 to 2.6 ± 6.5 episodes/month (−1.18, 95% CI 0.03–2.34, p = 0.046) in the 121–240 min group (Fig. 4f).
Discussion
We show here that a large difference in dose timing of insulin glargine U100 can be related to the frequency of hypoglycemia in type 2 diabetes. Approximately half of the study participants mistimed injections by >120 min on occasion and 10% of them mistimed injections by >240 min. A high BMI, drinking and smoking habits and living alone was associated with the larger variations in dose timing. Importantly, insulin degludec was found to decrease the frequency of hypoglycemia and improve glycemic control in participants with deviations in dose timing of >120 min.
Of the study participants with type 1 diabetes, 78.9% self-administered insulin within 120 min of the agreed-upon time. The variation in dose timing in these patients tended to be less than that in those with type 2 diabetes. However, analysis of our data revealed that a younger age was associated with a larger difference in dose timing of insulin glargine U100. Generally, younger type 1 diabetes patients tend to be less compliant to therapeutic regimens than their older counterparts [16, 27]. Our results suggest that healthcare professionals should therefore pay close attention to younger persons with diabetes regarding dose timing of basal insulin, especially if these patients have frequent hypoglycemia or poor glycemic control.
In our study participants with type 2 diabetes we found that a greater difference in dose mistiming of basal insulin was also associated with unhealthy lifestyle and family structure, with drinking and smoking habits and living alone significantly related to a larger difference in dose timing among this group. Furthermore, larger differences in dose timing were correlated with a higher body weight and higher BMI, suggesting that mistimed injection of basal insulin is linked to weight gain. Brod et al. also showed that persons with diabetes with numerous mistimed doses never or rarely ate meals at a regular time and were overweight or obese [28].
The main negative effect of a larger difference in dose timing, especially those >240 min, was an increase in the frequency of hypoglycemia in persons with type 2 diabetes. In our study the use of bolus insulin or any orally administered anti-diabetic drug was not related to frequency of hypoglycemia. The GAPP2 survey also revealed a statistically significant association between persons who missed, mistimed or reduced the number of basal insulin doses and episodes of self-treated hypoglycemia [29]. None of the participants in our study changed the insulin dose or missed a dose of insulin glargine U100, confirming that mistiming introduces risk of hypoglycemia. We suggest that healthcare professionals should communicate carefully with their patients and assess dose timing as a part of routine care. If their patients mistime basal insulin injection, the healthcare professionals need to encourage them to comply each day and to change their lifestyle as necessary. In addition, social support should be provided to persons living alone.
On the other hand, more user-friendly basal insulin is being considered. Ultra-long-acting insulins have recently been developed, notably insulin glargine U300 and insulin degludec. Glargine U300 has the benefits that its effects last for >24 h and dosing may occur up 3 h before or after the agreed time of administration [30–34]. The effects of insulin degludec last for 24–42 h at maximum [35, 36]; an attractive aspect of this drug is also its flexibility of injection timing [26, 37–41]. Dosing flexibility of insulin degludec by ±8 h from the usual time of administration can ensure the same adequate glycemic control and frequency of hypoglycemia as fixed dosing of the insulin [37]. In this study, insulin degludec was found to improve glycemic control and to reduce the occurrence of hypoglycemia in persons with diabetes whose variation in dose timing was >120 min. It is reported that the ability to dose insulin degludec flexibly can positively impact the health-related quality of life [42]. In addition, degludec is likely to be cost effective compared to other basal insulin analogs [43]. Thus, new-generation basal insulin may be helpful for all persons with diabetes.
Irregular dosing of basal insulin is believed to be related to two main factors, namely patient and medical factors, respectively. Patient factors, such as being too busy, forgetting, skipping meals, eating out, traveling, embarrassment about injecting in public and stress or emotional problems, can result in mistiming of insulin injections [15]. With respect to medical factors, healthcare professionals usually instruct persons with diabetes to inject basal insulin at mealtimes in combination with bolus insulin or oral medication, or at the time of waking, bedtime, morning or evening. Persons with diabetes are thus allowed leeway as to when to inject basal insulin. When healthcare professionals educate persons with diabetes at initiation of therapy with basal insulin, it is important to guide them to a regular life style and to individually determine fixed dose timing with an allowance of 2 h from the standpoint of better glycemic control, better body weight control and a lower hypoglycemia risk. On the other hand, when individuals are even then not able to inject basal insulin within 2 h of the agreed dose timing, insulin degludec or glargine U300 can be considered an option to prevent hypoglycemia and to improve glycemic control. Such patient-centered therapeutic strategies may improve not only glycemic control but also the quality of life of persons with diabetes [39].
There are limitations to our study. First, participants were recruited in a single hospital, so characteristics of the participants are biased accordingly. Second, the number of participants, especially of patients with type 1 diabetes, was small. Third, some hypoglycemia episodes as nocturnal hypoglycemia may have been missed because continuous glucose monitoring was not performed in the study. Fourth, the observation period was not long and may not have allowed sufficient time for the full effect of switching from insulin glargine U100 to degludec on glycemic control and hypoglycemia to be evaluated. Finally, changes in physical findings of the participants, such as body weight and blood pressure, were not measured after the switch from insulin glargine to insulin degludec. Further studies involving a larger number of persons with type 1 and type 2 diabetes over a longer period are needed.
Conclusion
A large difference in dose timing of basal insulin represents a risk of hypoglycemia and weight gain in persons with type 2 diabetes. Healthcare professionals should instruct persons with diabetes to inject basal insulin regularly at the agreed times. However, when required, a more flexibly timed basal insulin, such as insulin degludec, can be used to prevent hypoglycemia and weight gain.
References
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2015;38:140–9.
American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care. 2016;39[Suppl 1]:S52–9.
Tricco AC, Ashoor HM, Antony J, et al. Safety, effectiveness, and cost effectiveness of long acting versus intermediate acting insulin for patients with type 1 diabetes: systematic review and network meta-analysis. BMJ. 2014;349:g5459.
Fonseca VA, Haggar MA. Achieving glycaemic targets with basal insulin in type 2 diabetes by individualizing treatment. Nat Rev Endocrinol. 2014;10:276–81.
Owens DR, Matfin G, Monnier L. Basal insulin analogues in the management of diabetes mellitus: what progress have we made? Diabetes Metab Res Rev. 2014;30:104–19.
Benhamou PY, Gamier C, Debay I, et al. Basal insulin dose in 40 type 1 diabetic patients remains stable 1 year after educational training in flexible insulin therapy. Diabetes Metab. 2010;36:369–74.
Garg SK, Admane K, Freemantle N, et al. Patient-led versus physician-led titration of insulin glargine in patients with uncontrolled type 2 diabetes: a randomized multinational ATLAS study. Endocr Pract. 2015;21:143–57.
Evans M, Schumm-Draeger PM, Vora J, King AB. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011;13:677–84.
Sanofi-Aventis. Last update 2015. Lantus (insulin glargine U100) label Japan. 2017. http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/780069_2492416G2024_1_13. Accessed 29 Jan 2017.
Sanofi-Aventis. Last update 2016. Lantus (insulin glargine U100) label Europe. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000284/WC500036082.pdf. Accessed 29 Jan 2017.
Sanofi-Aventis. Last update 2015. Lantus (insulin glargine U100) label USA. 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021081s063lbl.pdf. Accessed 29 Jan 2017.
Novo Nordisk A/S. Last updated 2013. Levemir (insulin detemir) label Japan. 2017. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/620023_2492417G1030_1_09. Accessed 29 Jan 2017.
Novo Nordisk A/S. Last updated 2016. Levemir (insulin detemir) label Europe. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000528/WC500036662.pdf. Accessed 29 Jan 2017.
Novo Nordisk A/S. Last updated 2015. Levemir (insulin detemir) label USA. 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021536s031s051lbl.pdf. Accessed 29 Jan 2017.
Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–9.
Davies MJ, Gagliardino JJ, Gray LJ, Khunti K, Mohan V, Hughes R. Real-world factors affecting adherence to insulin therapy in patients with Type 1 or Type 2 diabetes mellitus: a systematic review. Diabet Med. 2013;30:512–24.
Xing D, Kollman C, Beck RW, et al. Juvenile diabetes research foundation continuous glucose monitoring study group. optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther. 2011;13:351–8.
Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–50.
Daitoku M, Honda I, Okumiya A, et al. Validity and reliability of the Japanese translated “The Summary of Diabetes Self-care Activities Measure”. J Japan Diabetes Soc. 2006;49:1–9 (in Japanese).
Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754–60.
Ishii H, Welch G, Jacobson A, et al. The Japanese version of the problem area in diabetes scale: a clinical and research tool for the assessment of emotional functioning among diabetes patients. Diabetes. 1999;48 (Suppl 1):A319.
World Health Organization. Info package: mastering depression in primary care. Frederiksborg, Denmark: WHO Regional Office of Europe, Psychiatric Research Unit; 1998.
Bradley C, Lewis KS. Measurements of psychological wellbeing and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med. 1990;7:445–51.
Awata S, Bech P, Yoshida S, et al. Reliability and validity of the Japanese version of the World Health Organization-five well-being index in the context of detecting depression in diabetic patients. Psychiatry Clin Neurosci. 2007;61:112–9.
Japan Diabetes Society. Practice guideline for the treatment for diabetes in Japan 2016. Tokyo: Nankodo; 2016.
Novo Nordisk A/S. Last updated 2016. Tresiba (insulin degludec) label Japan. 2017. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/620023_2492419G1021_1_08 Accessed 29 Jan 2017.
Gomes MB, Negrato CA. Adherence to insulin therapeutic regimens in patients with type 1 diabetes. A nationwide survey in Brazil. Diabetes Res Clin Pract. 2016;120:47–55.
Brod M, Rana A, Barnett AH. Adherence patterns in patients with type 2 diabetes on basal insulin analogues: missed, mistimed and reduced doses. Curr Med Res Opin. 2012;28:1933–46.
Brod M, Rana A, Barnett AH. Impact of self-treated hypoglycaemia in type 2 diabetes: a multinational survey in patients and physicians. Curr Med Res Opin. 2012;28:1947–58.
Goldman J, White JR Jr. New insulin glargine 300 U/mL for the treatment of type 1 and type 2 diabetes mellitus. Ann Pharmacother. 2015;49:1153–61.
Riddle MC, Bolli GB, Home PD, et al. Efficacy and safety of flexible versus fixed dosing intervals of insulin glargine 300 U/mL in people with type 2 diabetes. Diabetes Technol Ther. 2016;18:252–7.
Sanofi-Aventis. Last update 2015. Lantus XR (insulin glargine U300) label Japan. 2017. http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/780069_2492416G3020_1_02. Accessed 29 Jan 2017.
Sanofi-Aventis. Last update 2016. Toujeo (insulin glargine U300) label Europe. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000309/WC500047935.pdf. Accessed 29 Jan 2017.
Sanofi-Aventis. Last update 2015. Toujeo (insulin glargine U300) label USA. 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206538s000lbl.pdf. Accessed 29 Jan 2017.
Josse RG, Woo V. Flexibly timed once-daily dosing with degludec: a new ultra-long-acting basal insulin. Diabetes Obes Metab. 2013;15:1077–84.
Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14:944–50.
Kadowaki T, Jinnouchi H, Kaku K, Hersløv ML, Hyllested-Winge J, Nakamura S. Efficacy and safety of once-daily insulin degludec dosed flexibly at convenient times vs fixed dosing at the same time each day in a Japanese cohort with type 2 diabetes: a randomized, 26-week, treat-to-target trial. J Diabetes Investig. 2016;7:711–7.
Mathieu C, Hollander P, Miranda-Palma B, NN1250-3770 (BEGIN: Flex T1) Trial Investigators, et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98:1154–62.
Meneghini L, Atkin SL, Gough SC, NN1250-3668 (BEGIN FLEX) Trial Investigators, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36:858–64.
Novo Nordisk A/S. Last updated 2015. Tresiba (insulin degludec) label Eupopa. 2017 http://www.ema.europa.eu/docs/en_GB/document_library/EP.AR_-_Product_Information/human/002498/WC500138940.pdf. Accessed 29 Jan 2017.
Novo Nordisk A/S. Last updated 2015. Tresiba (insulin degludec) label USA. 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/203314lbl.pdf. Accessed 29 Jan 2017.
Evans M, Jensen HH, Bøgelund M, Gundgaard J, Chubb B, Khunti K. Flexible insulin dosing improves health-related quality-of-life (HRQoL): a time trade-off survey. J Med Econ. 2013;16:1357–65.
Evans M, McEwan P. Clinical and cost-effectiveness of insulin degludec: from clinical trials to clinical practice. J Comp Eff Res. 2015;4:279–86.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the international Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The authors thank the participants for their involvement in the study.
Disclosures
Akiko Nishimura, Haruna Fukushige, Yu Wang and Yanyan Liu declare that they have no conflict of interest. Shin-ichi Harashima has received a research grant from Sanofi, honoraria for speaking from Eli Lilly and consulting fees from Novo Nordisk. Kiminori Hosoda has received donations and honoraria for speaking from Sanofi and Eli Lilly. Nobuya Inagaki has received a research grant from Eli Lilly and a donation from Sanofi.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all participants for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/6C87F0605D73E2AB.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nishimura, A., Harashima, Si., Fukushige, H. et al. A Large Difference in Dose Timing of Basal Insulin Introduces Risk of Hypoglycemia and Overweight: A Cross-Sectional Study. Diabetes Ther 8, 385–399 (2017). https://doi.org/10.1007/s13300-017-0238-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-017-0238-7