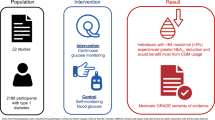
Abstract
Introduction
Individualizing glycemic targets to goals of care and time to benefit in persons with type 2 diabetes is good practice, particularly in populations at risk of hypoglycemia and adverse outcomes relating to the use of antihyperglycemics. Guidelines acknowledge the need for relaxed targets in frail older adults, but there is little guidance on how to safely deprescribe (i.e. stop, reduce or substitute) antihyperglycemics.
Methods
The purpose of this study was to synthesize evidence from all studies evaluating the effects of deprescribing versus continuing antihyperglycemics in older adults with type 2 diabetes. To this end, we searched MEDLINE, EMBASE, and Cochrane Library (July 2015) for controlled studies evaluating the effects of deprescribing antihyperglycemics in adults with type 2 diabetes. All such studies were eligible for inclusion in our study, and two independent reviewers screened titles, abstracts and full-text articles, extracted data, and evaluated risk of bias. Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment and a narrative summary were completed.
Results
We identified two controlled before-and-after studies, both of very low quality. One study found that an educational intervention decreased glyburide use while not compromising glucose control. The other reported that cessation of antihyperglycemics in elderly nursing home patients resulted in a non-significant increase in glycated hemoglobin (HbA1C). No significant change in hypoglycemia rate was found in the only study with this outcome measure.
Conclusions
There is limited evidence available regarding deprescribing antihyperglycemic medications. Adequately powered, high-quality studies, particularly in the elderly and with clinically important outcomes, are required to support evidence-based decision-making.
Protocol registration number
CRD42015017748.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to uncertainty regarding the benefits of intensive glycemic control in older persons, and the potential for harm from overtreatment in this population [1], organizations such as the Canadian Diabetes Association suggest a glycated hemoglobin (HbA1C) level of <8–8.5% in frail older adults as an appropriate target (consensus based) [2]. In older adults with diabetes, it is often unclear whether reducing glucose achieves meaningful risk reduction or prevents complications of hyperglycemia. Aggressive glycemic control has been questioned in the frail elderly and those with limited life expectancy, and adverse effects such as hypoglycemia are of concern [3, 4].
Despite efforts to reduce the risk of hypoglycemia in the elderly by relaxing glycemic control, there is little information on how to deprescribe, which includes reducing the dose or stopping/switching antihyperglycemic medications in order to individualize HbA1C targets. Clinicians are aware that relaxed glycemic targets may be appropriate in older patients, but they require guidance to assist with deprescribing [5]. To address this knowledge gap, we conducted a systematic review to identify studies that have evaluated the benefits and harms of deprescribing antihyperglycemics in adults with type 2 diabetes (T2DM).
Methods
Our protocol was registered in PROSPERO (CRD42015025727). We followed PRISMA guidelines [6]. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Sources and Searches
MEDLINE (1946 onward), EMBASE (1947 onward), and the Cochrane Library through to July 2015 were searched for relevant studies. The references of relevant studies were scanned. In addition, we searched clinicaltrials.gov, the World Health Organization Clinical Trials Registry, UpToDate, and Google Scholar. There was no limitation based on language. The search strategy can be found in Electronic Supplementary Material (ESM) Appendix 1.
Study Selection
Relevant studies included those involving patients aged ≥18 years who were taking antihyperglycemic medications for T2DM in any setting. For inclusion in our analysis, the following study designs were eligible, with no minimum follow-up time or sample size: randomized controlled trials, controlled before–after studies, interrupted time series, case–control studies, and prospective and retrospective cohort studies. Included studies compared the spectrum of deprescribing approaches (stopping drug treatment entirely, reducing dose, gradual tapering, or substitution) of at least one medication (insulin, metformin, sulfonylureas, thiazolidinediones, meglitinides, alpha-glucosidase inhibitors, dipeptidyl peptidase IV inhibitors, pramlintide, glucagon-like peptide-1 agonists) to continuing these medications. Included studies had to report on at least one of the following: hypoglycemia, falls, adverse drug reactions, frequency of blood glucose testing, blood glucose levels, HbA1C levels, pill burden, emergency room visits and hospitalizations, quality of life and patient satisfaction, length of stay in hospital, microvascular complications, macrovascular outcomes, polyuria, hyperglycemia, and/or sleep disturbances.
Data Extraction and Quality Assessment
Two independent reviewers screened titles and abstracts and evaluated full-text articles against eligibility criteria. The reviewers independently extracted data from eligible articles using a pilot-tested form. We extracted the following: year, journal, funding, study design, number of participants, proportion of male/female participants, comorbidities, duration of diabetes, concomitant medications, study medications, doses, frequency, duration and stopping/tapering/switching regimen and outcomes on benefits and harms of deprescribing.
Data Synthesis and Analysis
Two independent reviewers conducted risk of bias assessments for eligible studies using Cochrane’s ROBINS-I tool [7]. We conducted a narrative synthesis of results, using methods described in our registered protocol [8]. Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to assess quality of evidence [9].
Results
Study Selection
Our search generated 3458 titles after de-duplication. We evaluated 42 full-text articles and two articles met the eligibility criteria for qualitative synthesis [10, 11]. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram is displayed in Fig. 1.
Study Characteristics and Risk of Bias
The first of the controlled before-and-after studies which we identified as meeting the inclusion criteria was that of Aspinall et al. [11] who investigated deprescribing glyburide (discontinuing glyburide and either switching to an alternative agent or not adding additional medication to the therapeutic regimen) in American community-dwelling older adults via an educational intervention delivered to pharmacists (see Table 1). The second such study, by Sjöblom et al. [10], investigated the withdrawal of all antihyperglycemics (or a reduction of insulin) versus continuing antihyperglycemics in Swedish nursing home patients. The full study characteristics are outlined in Table 1.
Both studies were judged to be at serious risk of bias for all outcomes according to ROBINS-I tool [7] due to important problems with confounding, selection of participants, and deviations from intended interventions (ESM Appendices 2, 3).
Narrative Summary of Eligible Studies
In the study by Aspinall et al. [11], patients in the intervention group were more likely to stop glyburide [relative risk (RR) 1.28; 95% confidence interval (CI) 1.22–1.33] compared to those in the control group. The change in HbA1C levels from baseline to post-intervention was compared in patients who continued glyburide to those who discontinued glyburide and did not start another medication. No significant difference in HbA1C levels was found between the group of patients who discontinued glyburide and those who continued taking this medication (A1C increased by 0.04% in those who discontinued glyburide vs. 0.06% in those who continued; mean difference 0.02% lower; 95% CI: −0.16 to 0.12%). In addition, no significant difference was observed in the rates of hypoglycemia post-intervention between the intervention and control groups (RR 1.08; 95% CI 0.78–1.50). A change in HbA1C level was reported for patients (n = 999) who switched from glyburide to alternative medications, for whom HbA1C levels before and after the intervention were 7.29% [standard deviation (SD) 1.37%] and 7.33% (SD 1.41%), respectively. Of these patients, 87% (874/999) were switched to glipizide. A complete summary of findings is provided in ESM Appendix 4.
Sjöblom et al. [10] reported a non-significant increase in HbA1C level for the intervention group following deprescribing (mean difference 1.10%; 95% CI 0.56% lower to 1.64% higher). There was no significant difference in the risk of all-cause mortality for the deprescribing group compared to the control group (RR 0.74; 95% CI 0.29–1.87). A complete summary of the findings is provided in ESM Appendix 5.
Quality of Evidence
Based on the GRADE rating system, the quality of evidence for both studies was very low due to their non-randomized design and concerns surrounding the risk of bias and imprecision. GRADE evidence tables are given in ESM Appendices 4 and 5.
Discussion
Summary
Our systematic review identified two studies which assessed deprescribing antihyperglycemics in elderly patients. One trial involved a group of community-dwelling and predominantly male elderly patients with a baseline HbA1C level of approximately 7.2% [11]. Deprescribing glyburide in these patients does not appear to adversely affect glucose control, suggesting that an educational intervention aimed at pharmacists may reduce glyburide use without compromising glucose control. However, the quality of evidence of this study is very low. Although glyburide is associated with hypoglycemia and poses a higher risk than do other sulfonylureas [12], deprescribing of glyburide does not appear to reduce hypoglycemic events.
The second trial involved patients in 17 different nursing homes in Sweden [10]. Of these patients, 75% (24/32) remained in the intervention group after 3 months, with four patients withdrawn due to hyperglycemia. The results of this study demonstrate that frail elderly patients are often treated to well below the HbA1C targets and that deprescribing is possible in the majority of patients without a large impact on HbA1C levels (increase of 0.6% after 6 months in intervention group to an HbA1C level of 5.8%). Hypoglycemic events were not reported.
These studies suggest that the deprescribing of antihyperglycemics in older people is a feasible strategy and may not compromise blood glucose control or lead to clinically significant increases in HbA1C levels, albeit the published evidence is of very low quality.
Comparison to Existing Literature
A 2015 retrospective cohort study demonstrated that deintensification of diabetes therapy is attempted in around 20–30% of patients with low HbA1C levels [13]. However, this study did not report clinical outcomes of deintensification. A 2011 retrospective analysis of predominantly male elderly patients with renal impairment (creatinine clearance <50 mL/min) investigated the effect of switching from glyburide to glipizide (uncontrolled before–after study) [14]. Despite an increase in HbA1C level of 0.34% at 1 year, rates of hypoglycemia fell from 31 to 13%. These results suggest that hypoglycemia may be reduced following a switch to sulfonylureas with a lower risk of hypoglycemia and are consistent with meta-analysis data suggesting that glyburide carries an elevated risk of hypoglycemia compared to glipizide [12].
Future Research
Our findings signal a need for adequately powered high-quality antihyperglycemic deprescribing studies in the elderly population with T2DM—particularly in those populations affected by new guidelines recommending relaxed glycemic targets, with attention to the design of features that minimize the risk of bias. Randomized trials could be designed to compare deprescribing protocols to usual care since there is genuine clinical equipoise about the risks and benefits of these strategies, and this would be the least susceptible to selection bias. Non-randomized studies could be designed to compare those patients who have stopped or received tapered medication with those who continued, using established methods to minimize risk of bias due to confounding. Patients and their prescribers should engage in shared decision-making regarding whether to continue or deprescribe their antihyperglycemic medication. Different deprescribing approaches (e.g., tapering vs. abruptly discontinuing antihyperglycemics, the effect of deprescribing specific medications) should be studied. Patient-important outcomes, such as hypoglycemia rates, burden of treatment, quality of life, and function, as well as cost-effectiveness outcomes, should be measured.
Strengths and Limitations
We used rigorous systematic review methodology [6, 15] and GRADE to assess the quality of evidence. However, only two studies of very low quality were identified. We found limited outcome data in the eligible studies and a lack of patient-important outcomes. The Aspinall et al. study [11], while large, only provides evidence related to deprescribing glyburide; and may not apply to patients on other antihyperglycemics. Neither study [10, 11] provided practical information to assist clinicians in deprescribing.
Conclusion
The evidence needed to guide clinicians in helping patients achieve relaxed glycemic targets through deprescribing is currently lacking. While our systematic review suggests deprescribing approaches may be feasible and safe, we found no evidence of benefit or reduced harm. Adequately powered high-quality studies of deprescribing antihyperglycemics with patient-important outcomes are required to support evidence-based decision-making.
References
Lipska K, Ross J, Miao Y, Shah N, Lee S, Steinman M. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175(3):356–62.
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2013;37[Suppl 1]:S1–212.
Abdelhafiz A, Rodriguez-Manas L, Morley J, Sinclair A. Hypoglycemia in older people—a less well recognized risk factor for frailty. Aging Dis. 2015;6(2):156–67.
Lee S, Boscardin W, Cenzer I, Huang E, Rice-Trumble K, Eng C. The risks and benefits of implementing glycemic control guidelines in frail older adults with diabetes mellitus. J Am Geriatr Soc. 2011;59(4):666–72.
Caverly T, Fagerlin A, Zikmun-Fisher B, Kirsh S, Kullgren J, Prenovost K, et al. Appropriate prescribing for patients with diabetes at high risk of hypoglycemia. JAMA Intern Med. 2015;175(12):1994–6.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Black C, Thompson W, Lochnan H, McCarthy L, Rojas-Fernandez C, Shamji S, et al. Benefits and harms of deprescribing versus continuing antihyperglycemics for treatment of Type 2 Diabetes Mellitus: a systematic review protocol. PROSPERO. 2015;CRD4201502. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015025727.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
Sjöblom P, AndersTengblad Löfgren U-B, Lannering C, Anderberg N, Rosenqvist U, et al. Can diabetes medication be reduced in elderly patients? An observational study of diabetes drug withdrawal in nursing home patients with tight glycaemic control. Diabetes Res Clin Pract. 2008;82(2):197–202.
Aspinall SL, Zhao X, Good CB, Stone RA, Boresi J, Cox S, et al. Intervention to decrease glyburide use in elderly patients with renal insufficiency. Am J Geriatr Pharmacother. 2011;9(1):58–68.
Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30(2):389–94.
Sussman JB, Kerr E, Saini SD, Holleman RG, Klamerus ML, Min LC, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med. 2015;175(12):1–8.
Skoff R, Waterbury N, Shaw R, Egge J, Cantrell M. Glycemic control and hypoglycemia in Veterans Health Administration patients coverted from glyburide to glipizide. J Manag Care Pharm. 2011;17(9):664–71.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Higgins JP, Green S, editors. Cochrane Collab 2011. John Wiley & Sons; 2011.
Acknowledgments
Support for this review was provided by the Michel Saucier Endowed Chair in Geriatric Pharmacology, Health and Aging and a Canadian Institutes of Health Research Partnerships for Health System Improvement grant.
All authors named meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
The authors would like to acknowledge Tamara Rader, Patient Engagement Officer of the Canadian Agency for Drugs and Technologies in Health, for designing and executing the search strategy.
Disclosures
Wade Thompson has received honoraria for speaking on the topic of deprescribing at conferences. Heather Lochnan has participated as site investigator in industry-funded diabetes clinical trials that involve the use of medication used in the treatment of diabetes. Barbara Farrell has received research funding for the purposes of developing a related antihyperglycemic deprescribing guideline and has received travel support from the Canadian Pharmacists Association to present portions of this material at their annual meeting in June 2016. Cody Black, Vivian Welch, Lisa McCarthy, Carlos Rojas-Fernandez, Salima Shamji, and Ross Upshur have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/3947F0606828D009.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Black, C.D., Thompson, W., Welch, V. et al. Lack of Evidence to Guide Deprescribing of Antihyperglycemics: A Systematic Review. Diabetes Ther 8, 23–31 (2017). https://doi.org/10.1007/s13300-016-0220-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0220-9