Abstract
Introduction
Canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, has demonstrated sustained improvements in glycemic control and body weight reductions with treatment for up to 104 weeks in a broad range of patients with type 2 diabetes mellitus (T2DM).
Methods
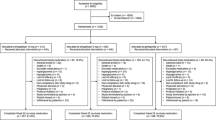
This was a post hoc analysis of individual patient data (N = 1450) from a randomized, double-blind, placebo-controlled, Phase 3 study comparing canagliflozin with glimepiride as add-on to metformin in patients with T2DM during a 52-week core period followed by a 52-week extension period. The number of patients who achieved a reduction from baseline in both HbA1c and body weight with canagliflozin 100 and 300 mg and glimepiride was assessed at Weeks 52 and 104. Safety was recorded as adverse events (AEs) during the study.
Results
Canagliflozin 100 and 300 mg provided durable glycemic improvements and body weight reductions compared with glimepiride over 104 weeks. At Week 52, the proportion of patients who achieved reductions in both HbA1c and body weight was 72.4% with canagliflozin 100 mg, 78.5% with canagliflozin 300 mg, and 26.8% with glimepiride; similar results were observed at Week 104 (65.5%, 71.1%, and 26.8% with canagliflozin 100 and 300 mg and glimepiride, respectively). The AE profile of canagliflozin was comparable to that observed in previous studies, with increased incidence of AEs related to the mechanism of SGLT2 inhibition (e.g., genital mycotic infections, urinary tract infections, and osmotic diuresis–related AEs) and a low risk of hypoglycemia.
Conclusion
More patients treated with canagliflozin experienced reductions in both HbA1c and body weight compared with glimepiride for up to 104 weeks. Canagliflozin was generally well tolerated in patients with T2DM when used in combination with metformin.
Clinical Trial Registration
ClinicalTrials.gov identifier, NCT00968812.
Funding
Janssen Research & Development, LLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current guidelines for the management of type 2 diabetes mellitus (T2DM) routinely recommend metformin as first-line therapy in those who are able to tolerate this agent [1]. However, there are numerous options for second-line therapies for use in combination with metformin in individuals unlikely to achieve their target HbA1c levels with metformin alone. Among these, sulfonylureas are a well-established drug class, but they are typically associated with weight gain and hypoglycemia [1, 2]. Many patients with T2DM are overweight or obese, and further increases in body weight may be detrimental to their well-being, particularly increasing the risk of cardiovascular disease and microvascular disease [3].
Sodium glucose co-transporter 2 (SGLT2) inhibitors are another therapeutic class recommended in current guidelines as an option for combination therapy with metformin and are associated with weight loss and a low risk of hypoglycemia [1]. Canagliflozin is an SGLT2 inhibitor approved for the treatment of adults with T2DM [4]. Canagliflozin reduces plasma glucose levels by lowering the renal threshold for glucose, thereby increasing urinary glucose excretion [5, 6]. In a head-to-head study of canagliflozin versus the sulfonylurea glimepiride as add-on to metformin in patients with T2DM, canagliflozin has demonstrated sustained improvements in glycemic control, together with sustained reductions in body weight, over 104 weeks [7, 8].
In addition to measuring HbA1c in patients with T2DM, there is increasing recognition of the value of using composite endpoints to evaluate T2DM treatments [9]. Since glycemic control and weight loss are beneficial for most patients with T2DM, this post hoc analysis evaluated the effect of canagliflozin versus glimepiride on reducing both HbA1c and body weight in patients with T2DM inadequately controlled with metformin for up to 104 weeks of treatment.
Methods
Study Design
This post hoc analysis was based on data from a 104-week, randomized, double-blind, active-controlled, non-inferiority, Phase 3 study in patients with T2DM inadequately controlled on metformin. Patients were randomized 1:1:1 to one of the following once-daily treatments: canagliflozin 100 mg, canagliflozin 300 mg, or glimepiride (titrated to a maximum of 6 or 8 mg). The study was conducted at 157 study centers in 19 countries and consisted of a 52-week, double-blind core treatment period, followed by a 52-week, double-blind extension period. Details of the study design have been reported previously [7, 8].
Briefly, patients were required to be aged 18–80 years and to have inadequately controlled T2DM (HbA1c between 7.0% and 9.5%) while receiving metformin at stable doses of ≥2000 mg/day (or ≥1500 mg/day if unable to tolerate higher doses) for at least 10 weeks. Exclusion criteria included a history of type 1 diabetes; a history of more than one severe hypoglycemia episode (within 6 months); repeated measurements of fasting plasma glucose or fasting self-monitored blood glucose of ≥15.0 mmol/L during the pre-treatment phase; an estimated glomerular filtration rate of <55 mL/min/1.73 m2 (or <60 mL/min/1.73 m2 if based on restriction of metformin use in local label) or serum creatinine concentrations of ≥124 μmol/L for men and ≥115 μmol/L for women; or were taking thiazolidinediones within 16 weeks before screening. Patients were not permitted to use any antihyperglycemic agents, except for metformin, unless pioglitazone was prescribed for rescue therapy. Use of insulin for ≥3 continuous or 7 total days within 3 months of screening was not permitted; however, insulin could be used on up to 2 occasions for no more than 7 consecutive days during the study.
Endpoints
Change in HbA1c and percent change in body weight from baseline were pre-specified study endpoints. Using individual patient data, the proportion of patients who achieved a decrease from baseline in both HbA1c (%) and body weight (kg) was assessed at Weeks 52 and 104. Safety was assessed based on adverse event (AE) reports. Documented hypoglycemia episodes included biochemically documented episodes (concurrent fingerstick glucose or plasma glucose ≤3.9 mmol/L with or without symptoms) and severe episodes (i.e., requiring the assistance of another individual or resulting in seizure or loss of consciousness).
Statistical Analyses
Analyses were performed using the modified intent-to-treat (mITT) population, which consisted of all randomized patients who received ≥1 dose of study drug. The last observation carried forward (LOCF) approach was used to impute missing data. Efficacy endpoints were analyzed using an analysis of covariance (ANCOVA) model, with treatment, stratification factors, and country as fixed effects and the corresponding baseline value as a covariate.
The least squares (LS) mean differences between groups and 2-sided 95% confidence intervals (CIs) were estimated for changes in HbA1c and body weight and for the proportion of patients achieving reductions in both HbA1c and body weight. Odds ratios (ORs) and 95% CIs for the achievement of reductions in both HbA1c and body weight were also estimated. P values are reported for the prespecified comparison of percent change in body weight with canagliflozin versus glimepiride at Week 52 only. Statistical testing was not prespecified for efficacy comparisons at Week 104 or for the post hoc composite endpoint analyses; therefore, no P values are reported. Statistical analyses were performed using SAS, version 9.2 (Cary, NC, USA).
Compliance with Ethics
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients included in the study.
Results
Patient Disposition and Baseline Characteristics
A total of 1450 subjects comprised the mITT and safety analysis sets; 71.0%, 66.6%, and 65.1% completed the 104-week, double-blind treatment period with canagliflozin 100 and 300 mg and glimepiride, respectively [8]. Baseline demographic and disease characteristics were comparable across treatment groups, with a mean age of 56.2 years, HbA1c of 7.8%, and body mass index (BMI) of 31.0 kg/m2 (Table 1). Mean metformin dose was 2177 mg/day; 62% of patients were taking metformin immediate release and 38% were taking metformin extended release. Among patients in the glimepiride group, 91.3% uptitrated over 104 weeks, with a mean maximum glimepiride dose of 5.8 mg and a mean final dose of 5.6 mg. Approximately 54% of subjects were considered to be obese (BMI ≥30 kg/m2), based upon National Institutes of Health criteria [10].
Efficacy
Across all groups, there was a reduction from baseline in HbA1c at 52 and 104 weeks (Table 2). The nadir in HbA1c was reached at Week 52 in both canagliflozin groups, with small increases thereafter; for glimepiride, the nadir in HbA1c was reached at Week 18, with a subsequent continual rise through Week 104 [8]. Body weight decreased over 52 weeks and then remained stable through Week 104 in both canagliflozin groups, while body weight increased over 52 weeks in the glimepiride group and then remained stable through Week 104 (Table 2) [8].
At Week 52, 72.4%, 78.5%, and 26.8% of patients achieved reductions in both HbA1c and body weight with canagliflozin 100 and 300 mg versus glimepiride, respectively (Fig. 1; Table 3). Odds for achieving this composite endpoint favored canagliflozin 100 and 300 mg versus glimepiride [ORs (95% CI) of 7.7 (5.7, 10.3) and 10.7 (7.9, 14.5), respectively]. At Week 104, 65.5%, 71.1%, and 26.8% of patients achieved reductions in both HbA1c and body weight with canagliflozin 100 and 300 mg and glimepiride, respectively (Fig. 2; Table 3). Odds for achieving this composite endpoint favored canagliflozin 100 and 300 mg versus glimepiride [ORs (95% CI) of 5.6 (4.2, 7.5) and 7.4 (5.5, 9.8), respectively].
Safety
Details of the safety of canagliflozin 100 and 300 mg versus glimepiride over 104 weeks have been previously reported [8]. Briefly, the overall incidence of AEs was 73.3%, 77.9%, and 78.4% with canagliflozin 100 and 300 mg and glimepiride, respectively; the incidence of AEs leading to discontinuation was 6.2%, 9.5%, and 7.3%, respectively, and the incidence of serious AEs was 9.7%, 9.7%, and 14.3%, respectively. Incidences of genital mycotic infections in men (9.5% and 9.1% vs 1.9%) and women (13.9% and 15.6% vs 2.7%), urinary tract infections (10.6% and 8.7% vs 6.8%), and AEs related to osmotic diuresis (5.8% and 6.6% vs 2.1%) were higher with canagliflozin 100 and 300 mg versus glimepiride. At Week 104, the incidence of documented hypoglycemia was lower with canagliflozin 100 and 300 mg versus glimepiride (6.8% and 8.2% vs 40.9%). The frequency of severe hypoglycemic episodes was 0.6% (n = 3), 0.2% (n = 1), and 3.3% (n = 16) with canagliflozin 100 and 300 mg and glimepiride, respectively.
Discussion
In this post hoc analysis, patients with T2DM inadequately controlled with metformin who were treated with canagliflozin were more likely to achieve improved glycemic control (as evidenced by lower HbA1c) and concomitant weight loss than those treated with glimepiride at 52 and 104 weeks. Canagliflozin was well tolerated with an AE profile comparable to that previously documented in other studies, including increased incidence of genital mycotic infections, urinary tract infections, and AEs related to osmotic diuresis [4]. In addition, canagliflozin was associated with lower rates of hypoglycemia compared with glimepiride, including a low risk of severe episodes, over 104 weeks.
Glycemic improvements, weight loss, and low risk of hypoglycemia have also been reported with the SGLT2 inhibitors dapagliflozin and empagliflozin in patients with T2DM on background metformin versus sulfonylurea [11–14]. Composite endpoint analyses of reduction in both HbA1c and body weight have been reported with dapagliflozin, but not empagliflozin, and results were generally consistent with the present study. In a 52-week study of dapagliflozin versus glipizide as add-on to metformin, a higher proportion of patients achieved reductions in both HbA1c and body weight with dapagliflozin versus glipizide (66.9% vs 21.3%) [15]. Overall, findings with canagliflozin and dapagliflozin demonstrate that SGLT2 inhibitors may provide better achievement of both HbA1c and weight loss compared with sulfonylureas in patients with T2DM.
A previous study suggested that ~40% of patients with T2DM who are receiving metformin monotherapy and add a sulfonylurea to their treatment regimen will gain weight in the year following intensification of therapy [3]. Additionally, with every increase in BMI category (from normal to severely obese), the rate of hospitalizations for cardiovascular disease, stroke, amputation, blindness, and end-stage renal disease rose [3]. Thus, in addition to improvements in glycemic control, the reductions in body weight provided by canagliflozin make it a promising treatment option for patients with T2DM who are overweight or obese. The benefits of weight loss on other diabetes-related outcomes with canagliflozin have been demonstrated in an analysis of pooled data from 4 randomized, double-blind, placebo-controlled trials in which ~15% of HbA1c lowering and ~40% of systolic blood pressure lowering was associated with weight loss; thus, each 1% reduction in body weight was associated with a 0.045% decrease in HbA1c and a 0.62 mmHg reduction in systolic blood pressure [16]. Indeed, a reduction of 5–10% in body weight in patients with T2DM is associated with beneficial effects on glycemia, blood pressure, high-density lipoprotein cholesterol (HDL-C), and triglycerides [17]. However, it is unknown whether these benefits will translate to improvement in long-term clinical outcomes, including cardiovascular disease. Patients with T2DM with pre-existing cardiovascular disease who had reductions in HbA1c, body weight, and blood pressure with the SGLT2 inhibitor empagliflozin were shown to have decreased risk of cardiovascular events and mortality [18]. The ongoing CANagliflozin cardioVascular Assessment Study (CANVAS; ClinicalTrials.gov Identifier: NCT01032629) and CANVAS-R (renal endpoints; NCT01989754) trials will evaluate the long-term efficacy and safety, including cardiovascular outcomes, of canagliflozin in patients with T2DM and a history or high risk of cardiovascular disease [19].
Conclusion
In summary, canagliflozin provided greater attainment of reductions in both HbA1c and body weight compared with glimepiride at Weeks 52 and 104, and was generally well tolerated in patients with T2DM when used as adjunctive therapy to metformin.
References
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9.
Belsey J, Krishnarajah G. Glycaemic control and adverse events in patients with type 2 diabetes treated with metformin + sulphonylurea: a meta-analysis. Diabetes Obes Metab. 2008;10 Suppl:1–7.
Gordon JP, Evans M, Puelles J, McEwan PC. Factors predictive of weight gain and implications for modeling in type 2 diabetes patients initiating metformin and sulfonylurea combination therapy. Diabetes Ther. 2015;6:495–507.
Rosenthal N, Meininger G, Ways K, Polidori D, Desai M, Qiu R, et al. Canagliflozin: a sodium glucose co-transporter 2 inhibitor for the treatment of type 2 diabetes mellitus. Ann N Y Acad Sci. 2015;1358:28–43.
Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–72.
Polidori D, Sha S, Mudaliar S, Ciaraldi TP, Ghosh A, Vaccaro N, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36:2154–61.
Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–50.
Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis D, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355–64.
Einarson TR, Diels J, Garg M, et al. Composite endpoints in trials of type-2 diabetes. Diabetes Obes Metab. 2014;16:492–9.
National Institutes of Health (NIH). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Evidence Report; NIH Publication No. 98-4083. 1998.
Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–22.
Nauck MA, Del Prato S, Durán-García S, Rohwedder K, Langkilde AM, Sugg J, et al. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add-on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes Metab. 2014;16:1111–20.
Del Prato S, Nauck M, Durán-García S, Maffei L, Rohwedder K, Theuerkauf A, et al. Long-term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add-on therapy to metformin in patients with type 2 diabetes: 4-year data. Diabetes Obes Metab. 2015;17:581–90.
Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700.
Rohwedder K, Nauck M, Durán-García S, et al. Combined HbA1c and weight reduction is achieved more frequently with add-on dapagliflozin than add-on glipizide in patients with type 2 diabetes inadequately controlled on metformin. Presented at: 73rd Scientific Sessions of the American Diabetes Association: Chicago; June 21–25, 2013.
Cefalu WT, Stenlöf K, Leiter LA, Wilding JPH, Blonde L, Polidori D, et al. Effects of canagliflozin on body weight and body composition, and relation to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58:1183–7.
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–6.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, et al. Rationale, design, and baseline characteristics of the canagliflozin cardiovascular assessment study (CANVAS)—a randomized placebo-controlled trial. Am Heart J. 2013;166:217–23.
Acknowledgments
This study was sponsored by Janssen Research & Development, LLC, Raritan, NJ, USA. Medical writing support, provided by Kimberly Dittmar, PhD, of MedErgy, and article processing charges were funded by Janssen Global Services, LLC.
L.A.L and G.L. contributed to the conduct of the study; and the acquisition, analysis, and interpretation of the data. U.V. and M.J.D. contributed to the analysis and interpretation of the data. W.C. contributed to the design and conduct of the study; and the acquisition, analysis, and interpretation of the data. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
L.A.L. has received research funding from, has provided continuing medical education on behalf of, and/or has served as a consultant to AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novo Nordisk, Pfizer, Roche, Sanofi, and Servier. G.L. has served as a consultant for Amgen, Boehringer Ingelheim, Janssen, and Sanofi. U.V. and W.C. are full-time employees of Janssen Research & Development, LLC. M.J.D. is a full-time employee of Janssen Scientific Affairs, LLC.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhance content for this article go to http://www.medengine.com/Redeem/1784F06070CA269F.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Leiter, L.A., Langslet, G., Vijapurkar, U. et al. Simultaneous Reduction in Both HbA1c and Body Weight with Canagliflozin Versus Glimepiride in Patients with Type 2 Diabetes on Metformin. Diabetes Ther 7, 269–278 (2016). https://doi.org/10.1007/s13300-016-0163-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0163-1