Abstract
Introduction
The objective of this study was to compare the clinical effectiveness of liraglutide with sitagliptin and assess the associated economic outcomes in patients with type 2 diabetes mellitus (T2DM) treated in real-world practice in the United States (US).
Methods
This retrospective cohort study used a large US claims database to identify patients with T2DM who initiated liraglutide or sitagliptin between January 2010 and December 2012. Adults (≥18 years old) with persistent use of therapy for ≥3 months were included. Changes in glycated hemoglobin A1c (A1C) and the proportion of patients achieving A1C targets (≤6.5% and <7%) were examined at 6-month follow-up. Diabetes-related total, medical, and pharmacy costs over the follow-up period were assessed. Multivariable regression models were used to estimate the outcomes associated with liraglutide relative to sitagliptin, adjusting for differences in patient demographics and clinical characteristics.
Results
The study included 1,465 patients with T2DM who initiated liraglutide (N = 376) or sitagliptin (N = 1,089) (mean age [standard deviation (SD)]: 54 [8.9] vs. 58 [10.8] years; 43.9% vs. 61.8% males; both P < 0.01). After controlling for confounding factors, liraglutide patients experienced 0.31% points greater reduction in A1C (0.95% vs. 0.63% points; P < 0.01) at 6-month follow-up than sitagliptin patients and were more likely to reach A1C targets of ≤6.5% (odds ratio [OR]: 2.00; P < 0.01) and <7% (OR: 1.55; P < 0.01). Liraglutide patients had $994 lower mean diabetes-related medical costs ($1,241 vs. $2,235; P < 0.01), but $544 higher diabetes-related pharmacy costs ($2,100 vs. $1,556; P < 0.01) during the follow-up. No difference was found in the total mean diabetes-related costs between the two cohorts.
Conclusion
Liraglutide showed greater improvement in glycemic outcomes than sitagliptin among adult patients with T2DM in real-world clinical practice. Although diabetes-related pharmacy costs for patients using liraglutide were higher compared with sitagliptin, these were offset by significantly lower diabetes-related medical costs, resulting in similar total diabetes-related costs between the two treatment groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes affects more than 8.3% of the total population and is the seventh leading cause of death in the United States (US) [1]. The total estimated direct and indirect costs of treating diabetes in the US were $176 billion and $69 billion, respectively, in 2012 [2]. Type 2 diabetes mellitus (T2DM) accounts for 90–95% of all diagnosed cases of diabetes [1].
The key to managing diabetes is optimal glycemic control, while minimizing the risk of experiencing hypoglycemia, which is associated with significant reduction in the development of microvascular and macrovascular complications [3–5]. While lifestyle modifications such as healthy eating and exercise can help control glycemia, pharmacological agents remain the mainstream treatment to maintain target glucose levels, especially due to the progressive nature of diabetes. Incretin-based therapies for the management of T2DM are effective in lowering glycated hemoglobin A1c (A1C) with a low risk of hypoglycemia [6–8]. These therapies, including injectable glucagon-like peptide-1 (GLP-1) receptor agonists (such as liraglutide [Victoza®; Novo Nordisk A/S, Bagsvaerd, Denmark]) and oral dipeptidyl peptidase-4 (DPP-4) inhibitors (such as sitagliptin [Januvia®; Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA]), have different mechanisms of actions in increasing insulin secretion and decreasing glucagon secretion [9].
Head-to-head trials of liraglutide versus sitagliptin have shown that liraglutide is superior to sitagliptin for glycemic control and weight loss [10–12]. Patients on liraglutide also reported greater treatment satisfaction than those using sitagliptin [13]. However, there are few studies assessing the real-world comparative effectiveness of these drugs. The economic implication of the clinical effectiveness of liraglutide versus sitagliptin is unknown in the US. This study aims to address this knowledge gap by comparing the real-world clinical and economic outcomes among persistent liraglutide and sitagliptin patients for the treatment of T2DM.
Methods
Data Source
This retrospective observational study utilized data from the Truven Health MarketScan® (Truven Health Analytics Inc., Ann Arbor, MI, USA) Commercial and Medicare Supplemental Insurance Databases. The databases contain administrative claims and eligibility records for over 30 million commercially insured individuals (i.e., working age adults and their dependents) and 3 million enrollees in Medicare supplemental plans. The data represent the healthcare experience of employees, dependents, and retirees with primary or Medicare supplemental coverage through privately insured health plans. In addition, the Truven Health MarketScan® Lab database, which contains 32.6 million lab test results for approximately 1.9 million unique privately insured patients, was linked to the healthcare claim databases on a patient level. Data from July 2009 through December 2012 were used in this study. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Sample Selection
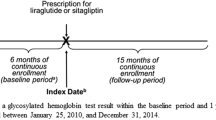
The study sample included patients who filled prescriptions for either liraglutide or sitagliptin between January 1, 2010 and December 31, 2012. The first pharmacy claim for liraglutide or sitagliptin defined the index therapy and the index date. Patients were required to be at least 18 years of age at the index date and have continuous health plan enrollment for at least 6 months prior to (washout period) and 6 months after (follow-up period) the index date. Patients were excluded if they (1) filled a prescription for any GLP-1 receptor agonists or any DPP-4 inhibitors during the washout period, (2) had type 1 diabetes (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] codes: 250.x1 or 250.x3), polycystic ovarian syndrome (ICD-9-CM code 256.4), pregnancy, or gestational diabetes during the washout or follow-up period.
The study analyzed outcomes from patients who continuously used the index therapy for at least 3 months (i.e., persistent users) to reduce the potential confounding from the use of other medications [14]. Treatment discontinuation was defined as a period of at least 90 consecutive days without a prescription fill of the index therapy. Persistent patients with at least one A1C measure in 45 days prior to 7 days after the index date and at least one A1C measure in ±45 days around the end of 6-month follow-up period were considered.
Demographic and Clinical Characteristics
Demographic characteristics assessed were age, gender, geographic region (Northeast, North Central, South, and West), metropolitan area status, health plan type (i.e., health maintenance organization, preferred provider organization, and others), and index year of treatment initiation. Baseline clinical characteristics included type of provider for the index therapy (primary care physician, endocrinologists, and other types) and several measures identified over the 6-month washout period: Charlson Comorbidity Index adapted to predict healthcare costs [15], the occurrence of common diabetes-related complications and comorbidities (retinopathy, nephropathy, neuropathy, cerebrovascular, cardiovascular, peripheral vascular disease, depression, obesity, hypertension, and hyperlipidemia) identified via ICD-9-CM codes [16], use of oral anti-diabetics (metformin, sulfonylurea, thiazolidione, and others) and insulin, and occurrence of severe hypoglycemia. Severe hypoglycemic events were identified by both ICD-9-CM codes [17] and recorded fasting plasma glucose level of less than or equal to 40 mg/dL [18].
Clinical and Economic Outcomes
Glycemic outcomes at 6 months of follow-up were measured by the following clinical endpoints: absolute change in A1C from baseline and A1C goal attainment of ≤6.5% and <7% as recommended by the American Association of Clinical Endocrinologists [19] and American Diabetes Association [20], respectively. The follow-up A1C measure was defined as the value closest to day 180 post-index within a ±45-day window, while baseline A1C was defined as the value closest to the index date within a window of 45 days prior to 7 days after the index date.
Economic outcomes assessed were total healthcare costs related to T2DM over the 6 months of follow-up, stratified by diabetes-related medical costs and pharmacy costs for anti-diabetic medications. These costs were identified from medical claims with a primary or secondary diagnosis code for diabetes (ICD-9-CM 250.xx), and pharmacy claims for oral anti-diabetic medication, non-insulin injectables (exenatide or liraglutide), and insulin. All costs were adjusted to 2013 values based on Consumer Price Index Medical Component [21].
Statistical Analysis
The study measures between treatment groups were compared using Student’s t test, Chi-square test, and Wilcoxon rank-sum test for continuous, categorical, and cost variables, respectively. Multivariable regression models were used to assess the association between the index therapy (liraglutide or sitagliptin) and the outcomes. The model specifications were based on the distribution of the outcomes. Absolute changes in A1C were assessed using ordinary least square regression, A1C goal attainment was assessed using logistic regression, and economic outcomes (total, medical, and pharmacy costs related to T2DM) were assessed using generalized linear models with a log link and gamma distribution. All regression models adjusted for baseline patient characteristics, including demographics and clinical characteristics, baseline A1C, and total healthcare costs related to T2DM during the 6-month washout period. Adjusted ratios of the outcomes associated with liraglutide and with sitagliptin were reported, including the odds ratio (OR) for the A1C goal attainment and the cost ratio (CR) for the economic outcomes. Adjusted values of the outcomes were calculated from the regression results using the method of recycled predictions, in which the adjusted outcomes were predicted twice for each patient in the sample, once assuming liraglutide and the other time assuming sitagliptin as the index therapy, respectively [22]. Data were compiled and analyzed using SAS (version 9.4, SAS Institute Inc., Cary, NC).
Results
A total of 444,651 adults filled prescriptions for liraglutide or sitagliptin between January 1, 2010 and December 31, 2012. After applying inclusion/exclusion criteria, the final study sample included 1,465 patients with baseline and follow-up A1C measures, of whom 376 were liraglutide patients and 1,089 were sitagliptin patients (Fig. 1). Patients initiating liraglutide differed significantly from those initiating sitagliptin in terms of characteristics. Liraglutide patients were younger (mean age [standard deviation (SD)]: 54 [8.9] years vs. 58 [10.8] years; P < 0.01) and were less likely to be males (43.9% vs. 61.8%; P < 0.01) than sitagliptin patients (Table 1). Liraglutide patients were more likely to obtain their prescriptions from endocrinologists than sitagliptin patients (15.7% vs. 4.4%; P < 0.01; Table 2). In addition, patients initiating liraglutide were less likely to have cardiovascular diseases (7.5% vs. 12.2%; P = 0.01) or nephropathy (4.8% vs. 8.2%; P = 0.03), less likely to use sulfonylurea (33.2% vs. 49.8%; P < 0.01), but more likely to use insulin during the washout period (35.4% vs. 16.9%; P < 0.01). Liraglutide patients had lower A1C (mean [SD]: 7.90% [1.7] vs. 8.18% [1.6]; P < 0.01) and lower total T2DM-related costs ($1,589 [2,784] vs. $2,049 [6,646]; P < 0.01) than sitagliptin patients at baseline. The baseline pharmacy costs for anti-diabetic medication were similar between the two groups.
Unadjusted outcomes for liraglutide patients were more favorable than for sitagliptin patients. Liraglutide patients experienced higher A1C reductions at 6-month follow-up (0.85% points [1.5] vs. 0.66% points [1.5]; P = 0.04) and were more likely to reach targets of A1C ≤6.5% (41.7% vs. 24.4%; P < 0.01) and A1C <7.0% (56.4% vs. 42.1%; P < 0.01; Table 3) than sitagliptin patients. Liraglutide patients also had lower total T2DM-related costs ($3,114 [4,029]) vs. $3,784 [9,251]); P < 0.01) than sitagliptin patients over the 6 months of follow-up despite higher pharmacy costs for anti-diabetic medication ($2,016 [1,547] vs. $1,580 [978]; P < 0.01).
Multivariable regression models were used to adjust for differences in baseline patient characteristics, including demographic and clinical characteristics, baseline A1C, and total healthcare costs related to T2DM during the 6-month washout period. These regression results were then used to predict the adjusted outcomes. The adjusted reduction of A1C at 6-month follow-up from baseline was predicted to be on average 0.31% points higher for liraglutide patients than sitagliptin patients (0.95% points vs. 0.63% points; P < 0.01; Table 4). Correspondingly, a higher proportion of liraglutide patients achieved of a target A1C ≤6.5% (OR: 2.00; P < 0.01) as well as target A1C <7% (OR: 1.55; P < 0.01) than sitagliptin patients at 6-month follow-up. Adjusted T2DM-related medical costs were 44% lower (CR: 0.56; P < 0.01) and pharmacy costs were 35% higher (CR: 1.35; P < 0.01) for liraglutide patients than for sitagliptin patients. Adjusted T2DM-related medical costs were $994 lower ($1,241 vs. $2,235; P < 0.01) and adjusted pharmacy costs were $544 higher ($2,100 vs. $1,556; P < 0.01) for liraglutide patients than for sitagliptin patients. The difference in the adjusted total diabetes-related costs was not statistically significant between the two therapies. Few other demographic and clinical characteristics were also statistically significantly associated with the clinical and economic outcomes (see the Appendix in electronic supplementary material).
Discussion
This is the first study to provide integrated evidence on the clinical effectiveness and the associated economic impact of treatment with liraglutide versus sitagliptin in real-world clinical practice in the US. In this intent-to-treat study, multivariable regressions were used to adjust for the differences in baseline patient demographics (such as age and gender) and clinical characteristics (such as baseline A1C level and diabetes-related costs) between patients initiating liraglutide and sitagliptin. Liraglutide patients showed significantly greater reduction in A1C and were more likely to achieve A1C targets than sitagliptin patients at 6 months of follow-up. The improved glycemic control associated with liraglutide compared with sitagliptin had economic implications. Despite higher pharmacy costs, liraglutide patients had significantly lower medical costs related to the management of T2DM over the 6 months follow-up compared with sitagliptin patients. No significant difference was found in the total diabetes-related costs between the treatment groups.
The clinical results of this study are consistent with the previously published findings from real-world practice. The study by Lee et al. [23] compared glycemic outcomes at 6 months of follow-up among adult patients with T2DM who initiated liraglutide, exenatide, or sitagliptin. Their study demonstrated that liraglutide patients had 0.40% points greater reduction in mean A1C (1.08% points vs. 0.68% points; P < 0.01) and a higher proportion attaining A1C target of <7% (64.4% vs. 49.4%; P < 0.01) at 6 months of follow-up than sitagliptin patients [23]. Another study, based on T2DM patients who were treated with at least 3 months of incretin-based therapies in Wales, UK, reported that the reduction in mean A1C from baseline was 0.44% points greater (1.23% points vs. 0.79% points; P < 0.05) among patients using liraglutide versus those using DPP-4 inhibitors [24].
The results from our real-world study support the trial findings that liraglutide is superior to sitagliptin in improving glycemic outcomes [10–12, 25]. Our study showed that patients with T2DM who initiated liraglutide had a greater reduction in A1C and a higher proportion of patients attained A1C targets of ≤6.5% and <7% compared with patients who initiated sitagliptin. A recent randomized parallel-group, open-label clinical trial compared the efficacy and safety of liraglutide versus sitagliptin at 26 weeks of treatment among adults with T2DM with inadequate glycemic control on metformin (N = 665; mean baseline A1C: 8.5%) [11]. In this trial, mean A1C reductions for liraglutide 1.2 mg (1.24% points) and 1.8 mg (1.50% points) were superior to those of sitagliptin (0.90% points) (both P < 0.01 compared with sitagliptin). A 26-week extension yielded similar results, with mean A1C reductions of 1.29% points, 1.51% points, and 0.88% points, respectively (both P < 0.01 compared with sitagliptin) [10]. The efficacy of liraglutide in this clinical trial, in terms of absolute mean reduction in A1C, is slightly higher than the clinical effectiveness found in our study (0.95% points adjusted mean reduction in A1C at 6-month follow-up) but the relative treatment effects of liraglutide versus sitagliptin are comparable. The higher absolute reduction in A1C associated with liraglutide in the clinical trial than in the current real-world study may reflect the stricter settings in clinical trials in terms of patient adherence and monitoring.
The greater improvement in clinical response associated with liraglutide versus sitagliptin may be linked to a lower economic burden in the treatment of T2DM. This study showed that savings in diabetes-related medical costs associated with the use of liraglutide offset the higher pharmacy costs of liraglutide. The economic outcomes in this study were assessed directly from insurance claims, and payment of the medical services and pharmacy prescriptions paid by the individual patients or the payers was considered. Previous studies assessed the cost-effectiveness of liraglutide versus sitagliptin by applying clinical trial efficacy data to economic models [13, 26]. Using clinical data from the randomized trial that compared the efficacy and safety of liraglutide versus sitagliptin at 26 and 52 weeks, the mean costs per patient to reach the composite endpoint of A1C <7% with no hypoglycemia or weight gain were notably lower for liraglutide than for sitagliptin ($4,225–4,855 lower at 26 weeks and $5,103–6,523 lower at 52 weeks; 2012 US dollars) [25].
Our study results imply that improved glycemic control in clinical practice is related to lower diabetes-related medical costs among liraglutide patients compared with sitagliptin patients. In clinical trials, liraglutide showed improved sustained body weight reduction and greater increase in beta cell function [10–12]. These factors, along with dose adjustment and the cost of the renal monitoring needed for the use of sitagliptin among patients with renal impairment [27, 28], may have impacted our findings. Future research could investigate how these additional factors are associated with the economic outcomes of the two treatment groups.
The interpretation of the results of this study should be made in light of the limitations associated with studies based on administrative claims data. First, the identification of persistent users of medication relied on dispensed prescriptions, without information of the actual drug consumption. However, in general, pharmacy claims are an accurate measure of prescription drug consumption [29, 30]. Second, due to the non-experimental nature of the data and the study design, unmeasured confounders like prescription bias may influence the results. Our study considered a wide range of covariates, including socio-demographic, clinical, and severity of illness measures to reduce the residual confounding due to factors like prescription bias. Third, although clinical trials determined the efficacy of liraglutide 1.2 mg separately from 1.8 mg, our study considered the overall use of liraglutide. It is challenging to calculate the dose of liraglutide in clinical practice using claims data since the National Drug Codes may not completely capture the clinical practice of dosing and titration, which may also vary over the considered follow-up period. Fourth, the database does not have body mass index or weight information available, so obesity was identified only by ICD-9-CM codes. This may result in under-reporting of the prevalence of obesity. Finally, the observational nature of the study design does not allow for causal inferences.
Conclusion
In real-world clinical practice in the US, liraglutide provides significantly greater improvements in clinical outcomes as compared with sitagliptin, without any increase in total diabetes-related costs over 6 months of follow-up. This information could be used to guide therapeutic decision makers to make cost-effective decisions for treatment of patients with T2DM.
References
American Diabetes A. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–80.
American Diabetes A. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36:1033–46.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Group TUPDSU. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53.
Gilbert MP, Pratley RE. Efficacy and safety of incretin-based therapies in patients with type 2 diabetes mellitus. Eur J Intern Med. 2009;20(Suppl 2):S309–18.
Russell S. Incretin-based therapies for type 2 diabetes mellitus: a review of direct comparisons of efficacy, safety and patient satisfaction. Int J Clin Pharm. 2013;35:159–72.
White J. Efficacy and safety of incretin based therapies: clinical trial data. J Am Pharm Assoc. 2003;2009(49 Suppl 1):S30–40.
Ahren B, Schmitz O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res. 2004;36:867–76.
Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65:397–407.
Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56.
Pratley RE, Nauck MA, Bailey T, et al. Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: a randomized, open-label trial. Diabetes Care. 2012;35:1986–93.
Davies M, Pratley R, Hammer M, Thomsen AB, Cuddihy R. Liraglutide improves treatment satisfaction in people with type 2 diabetes compared with sitagliptin, each as an add on to metformin. Diabet Med. 2011;28:333–7.
Maciejewski ML, Bryson CL, Wang V, Perkins M, Liu CF. Potential bias in medication adherence studies of prevalent users. Health Serv Res. 2013;48:1468–86.
Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61:1234–40.
Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23.
Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4.
Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–31.
Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540–59.
American Diabetes A. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11–66.
Bureau of Labor Statistics. Consumer Price Index. In: Consumer Price Indexes. 2014. Available from: http://www.bls.gov/cpi/. Accessed April 18, 2014.
Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–9.
Lee WC, Dekoven M, Bouchard J, Massoudi M, Langer J. Improved real-world glycaemic outcomes with liraglutide versus other incretin-based therapies in type 2 diabetes. Diabetes Obes Metab. 2014;16:819–26.
Evans M, McEwan P, O’Shea R, George L. A retrospective, case-note survey of type 2 diabetes patients prescribed incretin-based therapies in clinical practice. Diabetes Ther. 2013;4:27–40.
Langer J, Hunt B, Valentine WJ. Evaluating the short-term cost-effectiveness of liraglutide versus sitagliptin in patients with type 2 diabetes failing metformin monotherapy in the United States. J Manag Care Pharm. 2013;19:237–46.
Lee WC, Samyshkin Y, Langer J, Palmer JL. Long-term clinical and economic outcomes associated with liraglutide versus sitagliptin therapy when added to metformin in the treatment of type 2 diabetes: a CORE Diabetes Model analysis. J Med Econ. 2012;15(Suppl 2):28–37.
Novo Nordisk Inc. Victoza® (liraglutide [rDNA origin] injection), solution for subcutaneous use. Prescribing information. Version 6. Novo Nordisk A/S, DK-2880 Bagsvaerd, Denmark, Plainsboro, NJ: Novo Nordisk Inc; 2013. Available from: http://www.novo-pi.com/victoza.pdf.
Merck Sharp & Dohme Corp. Januvia® (sitagliptin) tablets. Prescribing information. Whitehouse Station, NJ: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc.; 2014. Available from: http://www.merck.com/product/usa/pi_circulars/j/januvia/januvia_pi.pdf.
Brown DW, Anda RF, Felitti VJ. Self-reported information and pharmacy claims were comparable for lipid-lowering medication exposure. J Clin Epidemiol. 2007;60:525–9.
Richardson K, Kenny RA, Peklar J, Bennett K. Agreement between patient interview data on prescription medication use and pharmacy records in those aged older than 50 years varied by therapeutic group and reporting of indicated health conditions. J Clin Epidemiol. 2013;66:1308–16.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Novo Nordisk Inc., Plainsboro, NJ, USA. AC made signification contributions to the analysis and interpretation of the data and wrote a significant portion of the manuscript. QL participated in the study design and critically reviewed and edited the manuscript. MH and JL contributed to the study conception and design and reviewed the manuscript. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Conflict of interest
QL is an employee of Evidera. AC is an employee of Evidera. Evidera provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In this salaried position, they work with a variety of companies and organizations and are precluded from receiving payment or honoraria directly from these organizations for services rendered. MH is an employee of Novo Nordisk Inc. and a shareholder of Novo Nordisk. JL is an employee of Novo Nordisk Inc. and a shareholder of Novo Nordisk. Novo Nordisk provided funding to Evidera for their role in the study and manuscript.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Li, Q., Chitnis, A., Hammer, M. et al. Real-World Clinical and Economic Outcomes of Liraglutide Versus Sitagliptin in Patients with Type 2 Diabetes Mellitus in the United States. Diabetes Ther 5, 579–590 (2014). https://doi.org/10.1007/s13300-014-0084-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-014-0084-9