Abstract
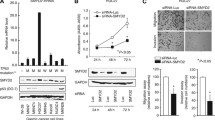
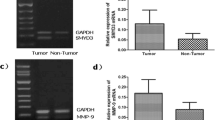
SET and MYND domain-containing protein 3 (SMYD3), a histone methyltransferase, plays a key function in the progression of human cancer. However, the role of SMYD3 in gastric carcinoma carcinogenesis has yet to be elucidated. This study aimed to determine the relationships of SMYD3 expression with clinicopathological characteristics and prognosis in gastric carcinoma. The expression of SMYD3 was detected by real-time quantitative reverse transcription PCR and Western blot in gastric carcinoma (GC) cell lines, normal gastric mucosa cell line, GC tissues, and adjacent non-tumor tissues. SMYD3 expression in tissue sections of 180 gastric carcinoma samples were evaluated using immunohistochemistry. The staining results were compared with clinicopathological characteristics and to the outcome of patients. The expression levels of SMYD3 messenger RNA (mRNA) and protein in GC tissues were both higher than those in adjacent non-tumor tissues (p < 0.05). SMYD3 mRNA and protein expression levels were higher in GC cell lines MKN28, SGC7901, and MGC803 than normal gastric mucosa cell line GES-1. SMYD3 expression in gastric carcinoma was significantly correlated with primary tumor size (p < 0.001), lymph node metastasis (p < 0.001), and TNM stage (p = 0.011). Degree of differentiation [hazard ratio (HR) = 5.113; p = 0.006], serosal invasion (HR = 2.074; p = 0.024), lymph node metastasis (HR = 1.354; p < 0.001), and SMYD3 expression (HR = 0.564; p = 0.004) were identified as the independent factors of the overall survival (OS) in all enrolled GC patients. For patients with positive lymph node metastasis, degree of differentiation (HR = 5.974; p = 0.015), lymph node metastasis (HR = 1.257; p < 0.001), and SMYD3 expression (HR = 0.529; p = 0.004) were the independent prognostic factors of the OS. SMYD3 performed an important function in the aggressiveness of gastric carcinoma and may act as a promising target for prognostic prediction.





Similar content being viewed by others
References
Jaskelioff M, Peterson CL. Chromatin and transcription: histones continue to make their marks. Nat Cell Biol. 2003;5:395–9.
Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15:172–83.
Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5.
Hamamoto R, Furukawa Y, Morita M, Iimura Y, Silva FP, Li M, et al. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat Cell Biol. 2004;6:731–40.
Xu S, Wu J, Sun B, Zhong C, Ding J. Structural and biochemical studies of human lysine methyltransferase Smyd3 reveal the important functional roles of its post-SET and TPR domains and the regulation of its activity by DNA binding. Nucleic Acids Res. 2011;39:4438–49.
Jakowlew SB, Breathnach R, Jeltsch JM, Masiakowski P. Chambon P sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984;12:2861–78.
Kunizaki M, Hamamoto R, Silva FP, Yamaguchi K, Nagayasu T, Shibuya M, Nakamura Y, Furukawa Y. The lysine 831 of vascular endothelial growth factor receptor 1 is a novel target of methylation by SMYD3. Cancer Res. 67:10759–10765.
Luo XG, Xi T, Guo S, Liu ZP, Wang N, Jiang Y, et al. Effects of SMYD3 overexpression on transformation, serum dependence, and apoptosis sensitivity in NIH3T3 cells. IUBMB Life. 2009;61:679–84.
Dong SW, Zhang H, Wang BL, Sun P, Wang YG, Zhang P. Effect of the downregulation of SMYD3 expression by RNAi on RIZ1 expression and proliferation of esophageal squamous cell carcinoma. Oncol Rep. 2014;32:1064–70.
Sponziello M, Durante C, Boichard A, Dima M, Puppin C, Verrienti A, et al. Epigenetic-related gene expression profile in medullary thyroid cancer revealed the overexpression of the histone methyltransferases EZH2 and SMYD3 in aggressive tumours. Mol Cell Endocrinol. 2014;392:8–13.
Chen LB, Xu JY, Yang Z, Wang GB. Silencing SMYD3 in hepatoma demethylates RIZI promoter induces apoptosis and inhibits cell proliferation and migration. World J Gastroenterol. 2007;13:5718–24.
Hamamoto R, Silva FP, Tsuge M, Nishidate T, Katagiri T, Nakamura Y, et al. Enhanced SMYD3 expression is essential for the growth of breast cancer cells. Cancer Sci. 2006;97:113–8.
Wang SZ, Luo XG, Shen J, Zou JN, Lu YH, Xi T. Knockdown of SMYD3 by RNA interference inhibits cervical carcinoma cell growth and invasion in vitro. BMB Rep. 2008;41:294–9.
Kim H, Heo K, Kim JH, Kim K, Choi J, An W. Requirement of histone methyltransferase SMYD3 for estrogen receptor-mediated transcription. J Biol Chem. 2009;284:19867–77.
Yuan Y. A survey and evaluation of population-based screening for gastric cancer. Cancer Biol Med. 2013;10:72–80.
Yang L. Incidence and mortality of gastric carcinoma in China. World J Gastroenterol. 2006;12:17–20.
Zhang XM, Zhou C, Gu H, Yan L, Zhang GY. Correlation of RKIP, STAT3 and cyclin D1 expression in pathogenesis of gastric cancer. Int J Clin Exp Pathol. 2014;7:5902–8.
He C, Xu J, Zhang J, Xie D, Ye H, Xiao Z, et al. High expression of trimethylated histone H3 lysine 4 is associated with poor prognosis in hepatocellular carcinoma. Hum Pathol. 2012;43:1425–35.
Cock-Rada AM, Medjkane S, Janski N, Yousfi N, Perichon M, Chaussepied M, et al. SMYD3 promotes cancer invasion by epigenetic upregulation of the metalloproteinase MMP-9. Cancer Res. 2012;72:810–20.
Ren TN, Wang JS, He YM, Xu CL, Wang SZ, Xi T. Effects of SMYD3 over-expression on cell cycle acceleration and cell proliferation in MDA-MB-231 human breast cancer cells. Med Oncol. 2011;28:S91–8.
Wang SZ, Luo XG, Shen J, Zou JN, Lu YH, Xi T. Knockdown of SMYD3 by RNA interference inhibits cervical carcinoma cells growth and invasion in vitro. BMB Rep. 2008;41:294–9.
Zou JN, Wang SZ, Yang JS, Luo XG, Xie JH, Xi T. Knockdown of SMYD3 by RNA interference down-regulates c-Met expression and inhibits cells migration and invasion induced by HGF. Cancer Lett. 2009;280:78–85.
Wang XQ, Miao X, Cai Q, Garcia-Barcelo MM. Fan STSMYD3 tandem repeats polymorphism is not associated with the occurrence and metastasis of hepatocellular carcinoma in a Chinese population. Exp Oncol. 2007;29:71–3.
Hundahl SA, Phillips JL, Menck HR. The national cancer data base report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth edition American joint committee on cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–32.
Abe N, Watanabe T, Suzuki K, et al. Risk factors predictive of lymph node metastasis in depressed early gastric carcinoma. Am J Surg. 2002;183:168–72.
Hochwald SN, Kim S, Klimstra DS, Brennan MF, Karpeh MS. Analysis of 154 actual 5-year survivors of gastric carcinoma. J Gastrointest Surg. 2000;4:520–5.
Deng J, Liang H, Sun D, Pan Y. The prognostic analysis of lymph node-positive gastric carcinoma patients following curative resection. J Surg Res. 2010;161:47–53.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 31470816).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Luo, X., Deng, J. et al. SMYD3 overexpression was a risk factor in the biological behavior and prognosis of gastric carcinoma. Tumor Biol. 36, 2685–2694 (2015). https://doi.org/10.1007/s13277-014-2891-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2891-z