Abstract
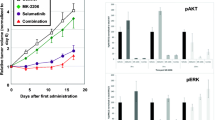
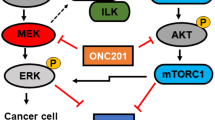
Although epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) cetuximab are used widely to treat KRAS wild-type metastatic colorectal cancer (mCRC), patients become resistant by various mechanisms, including KRAS, BRAF, and PIK3CA mutations, thereafter relapsing. AZD6244 is a potent, selective, and orally available MEK1/2 inhibitor. In this study, we investigated the mechanisms of AZD6244 alone or with BEZ235, an orally available potent inhibitor of phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR), in a KRAS and PIK3CA mutation CRC xenograft model. HCT116 (KRAS G13D, PIK3CA H1047R mutant) cells were subcutaneously injected into the nude mice. Mice were randomly assigned to treatment with vehicle, cetuximab, AZD6244, BEZ235, or AZD6244 plus BEZ235, for up to 3 weeks; then, all mice were sacrificed, and tumor tissues were subjected to Western blot analysis and immunohistochemical staining. AZD6244 or BEZ235 slightly inhibit tumor growth of HCT116 xenografts, and the combination treatment markedly enhanced their antitumor effects. However, cetuximab had no effect on tumor growth. Western blot analysis and immunohistochemical staining revealed that treatment with AZD6244 or BEZ235 could significantly reduce the phosphorylation level of ERK1/2 or AKT in HCT116 tumor tissues. More interesting, the antiangiogenic effects were substantially enhanced when the agents were combined which may due to the reduced expression of VEGF and matrix metallopeptidase-9 (MMP-9) in tumor tissues. These results suggest that the combination of a selective MEK inhibitor and a PI3K/mTOR inhibitor was effective in CRC harboring with KRAS and PIK3CA mutations. The mechanisms of synergistic antitumor effects may be due to antiangiogenesis.





Similar content being viewed by others
References
Malvezzi M, Arfe A, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2011. Ann Oncol. 2011;22:947–56.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–7.
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64.
Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1–9.
Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74.
Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–65.
Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–10.
Samuels Y, Wan ZG, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554.
De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–62.
Davies BR, Logie A, Jennifer S, McKay, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellularsignal-regulated kinase kinase 1/2 kinases: mechanism ofaction in vivo, pharmacokinetic/pharmacodynamicmodels relationship, and potential for combination in preclinical. Mol Cancer Ther. 2007;6:2209–19.
Sano T, Takeuchi S, Nakagawa T, Ishikawa D, Nanjo S, Yamada T, et al. The novel phosphoinositide 3-kinase-mammalian target of rapamycin inhibitor, BEZ235, circumvents erlotinib resistance of epidermal growth factor receptor mutant lung cancer cells triggered by hepatocyte growth factor. Int J Cancer. 2013;133:505–14.
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71.
Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–46.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics 2009. CA Cancer J Clin. 2010;59:225–49.
Shimizu T, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Smith LS, et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res. 2012;15(18):2316–25.
Britten CD. PI3K and MEK inhibitor combinations: examining the evidence in selected tumor types. Cancer Chemother Pharmacol. 2013;71:1395–409.
Solit DB, Garraway LA, Pratilas CA, Sawal A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2005;439:358–62.
Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64.
Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–10.
Fremin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol. 2010;3:8–14.
Bennouna J, Lang I, Valladares-Ayerbes M, Boer K, Adenis A, Escudero P, et al. A Phase II, open label, randomised study to assess the efficacy and safety of the MEK1/2 inhibitor AZD6244 (ARRY-142886) versus capecitabine monotherapy in patients with colorectal cancer who have failed one or two prior chemotherapeutic regimens. Invest New Drugs. 2011;29:1021–8.
Hoeflich KP, O’Brien C, Boyd Z, Cavet G, Guerrero S, Jung K, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15:4649–64.
Wee S, Jagani Z, Xiang KX, Loo A, Dorsch M, Yao YM, et al. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–93.
Engelman JA. Targeting PI3K signaling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62.
Liu Y, Yang Y, Ye YC, Shi QF, Chai K, Tashiro S, et al. Activation of ERK-p53 and ERK-mediated phosphorylation of Bcl-2 are involved in autophagic cell death induced by the c-Met inhibitor SU11274 in human lung cancer A549 cells. J Pharmacol Sci. 2012;118:423–32.
Velho S, Oliveira C, Ferreira A, Ferreira AC, Suriano G, Schwartz Jr S, et al. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur J Cancer. 2005;41:1649–54.
Prenen H, De Schutter J, Jacobs B, De Roock W, Biesmans B, Claes B, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184–8.
Funding
The study was funded by molecular mechanism study of liver metastasis of colorectal cancer affected by EMT under Wnt path adjusted by NLK 81272670.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
E, J., Xing, J., Gong, H. et al. Combine MEK inhibition with PI3K/mTOR inhibition exert inhibitory tumor growth effect on KRAS and PIK3CA mutation CRC xenografts due to reduced expression of VEGF and matrix metallopeptidase-9. Tumor Biol. 36, 1091–1097 (2015). https://doi.org/10.1007/s13277-014-2667-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2667-5