Abstract
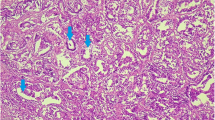
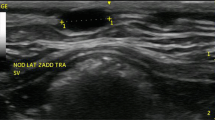
In this study, the frequency of different types of mammary masses and their relationship with cytohistopathologic changes was investigated and data on history, macroscopic description, clinical examination and treatment were collected. To determine the prevalence and types of cytohistopathologic changes, mammary glands from 12 female cats were evaluated. The mean age of cats at the time of diagnosis was 11.5 ± 1.9 years (range 4–14 years), the mean gross size of the masses was 3.1 ± 2.4 cm, 4/12 (33.3 %) masses were ≤3.0 cm in diameter, and the maximum diameter of the largest mass had a median of 5 cm, with a range of diameter of 6 × 5 × 4 cm. Moreover, the preferential localization of mammary masses was the abdominal lobes (%50) and thoracic lobes (%33.3), and inguinal lobes (%16.7 of cases). Furthermore, two cases of the inguinal masses affected the caudo-inguinal lobe, six cases caudo-abdominal lobe, and thoracic masses were found in four cases. Eventually, six cases (%50) of masses were found in the right mammary lobes and six cases (%50) in the left mammary lobes. The majority of the masses revealed elastic (%50 of cases), hard (%25 of cases), or soft (%25 of cases) consistency. In the present study, according to the criteria of the veterinary and the medical WHO classification system, of the 12 cats with the cytohistopathological features of six (50 %) cases qualified abscess, 3 (25 %) cases as cystic hyperplasia and 3 (25 %) cases were called situ carcinoma. Whereas, all hyperplastic lesions (case nos. 7–9 and ranging in size from, 1 to >4 cm3) and carcinomas in situ lesions (case nos. 10–12 and ranging in size from, 1 to >3 cm3) were found incidentally upon routine cytohistology. Other lesions were observed grossly and removed either at surgery (case nos. 1–6). Finally, the cats were treated with unilateral lumpectomy (3 cases) and also, nine (75 %) cases had subsequent drainage, 3 (25 %) of which showed cystic hyperplasia and 6 (50 %) showed abscess on subsequent histopathological evaluation. Therefore, a correct diagnosis must be established quickly, and treatment must be instituted rapidly when alteration is noted in the mammary glands.







Similar content being viewed by others
References
Murphy S. Mammary tumors in cats – causes and practical management. Conference proceedings of the European Society of Feline Medicine - ESFM Feline Symposium, 1st April 2009, Birmingham, UK: 2009: 11–15.
Dixon JM. Breast infection. In: Dixon JM, editor. ABC of breast diseases. 3rd ed. Oxford: Blackwell; 2006. p. 19–23.
Hahn KA, Adams WH. Feline mammary neoplasia: biological behaviour, diagnosis a treatment alternatives. Feline Pract. 1997;25:5–11.
Misdorp W, Else RW, Helmén E, Lipscomb TP. Histological classification of mammary tumors of the dog and cat. Washington: Armed Forces Institute of Pathology and World Health Organization; 1999. p. 3–6.
Rutteman GR, Withrow SJ, MacEwen EG. Tumors of the mammary gland. In: Withrow SW, MacEwen EG, editors. Small animal clinical oncology. 3rd ed. Philadelphia: WB Saunders Co; 2001. p. 467–73.
Millanta F, Lazzeri G, Mazzei M, Vannozzi I, Poli A. MIB-1 labelling index in feline dysplastic and neoplastic mammary lesions and its relationship with postsurgical prognosis. Vet Pathol. 2002;39:120–6.
Seixas Travassos MA. [Feline mammary lesions: a contribute to its biopathological characterization] In portuguese. PhD thesis, Univ. de Trás-os-Montes e Alto Douro. 2006; 194.
Baker R, Lumsden JH. The mammary gland. In: Baker R, Lumsden JH, editors. Color atlas of cytology of the dog and cat. St Louis: Mosby; 2000. p. 253–62.
Shafiee R, Javanbakht J, Atyabi N, Kheradmand P, Kheradmand D, Bahrami A, et al. Diagnosis, classification and grading of canine mammary tumours as a model to study human breast cancer: an clinico-cytohistopathological study with environmental factors influencing public health and medicine. Cancer Cell Int. 2013;13:79.
Shafiee R, Javanbakht J, Atyabi N, Bahrami A, Kheradmand D, Safaei R, et al. Comparative value of clinical, cytological, and histopathological features in feline mammary gland tumors; an experimental model for the study of human breast cancer. Diagn Pathol. 2013;8:136.
Bibbo M. Comprehensive cytopathology. 2nd ed. Philadelphia: WB Saunders Company; 1997. p. 413–44.
Rosen PP, Oberman HA. Tumors of mammary gland. Washington: Armed Forces Institute of Pathology; 1993. p. 390.
Nieto A, Peña L, Pérez-Alenza MD, Sánchez MA, Flores JM, Castaño M. Immunohistologic detection of estrogen receptor alpha in canine mammary tumors: clinical and pathologic associations and prognostic significance. Vet Pathol. 2000;37(3):239–47.
Sarli G, Preziosi R, Benazzi C, Castellani G, Marcato PS. Prognostic value of histologic stage and proliferative activity in canine malignant mammary tumors. J Vet Diagn Invest. 2002;14(1):25–34.
Yang WY, Liu CH, Chang CJ, Lee CC, Chang KJ, Lin CT. Proliferative activity, apoptosis and expression of oestrogen receptor and Bcl-2 oncoprotein in canine mammary gland tumors. J Comp Pathol. 2006;134(1):70–9.
Morris JS. Improving the diagnosis and treatment of canine mammary tumours: immunohistochemical markers as prognostic tools. Vet J. 2010;184(1):3–4.
Hayes HM, Milne KL, Mandell CP. Epidemiological features of feline mammary carcinoma. Vet Rec. 1981;108:476–9.
MacEwen EG, Hayes AA, Harvey HJ, Patnaik AK, Mooney S, Passe S. Prognostic factors for feline mammary tumors. J Am Vet Med Assoc. 1984;185:201–4.
Tomlinson MJ, Barteaux L, Ferns LE, Angelopoulous E. Feline mammary carcinoma: a retrospective evaluation of 17 cases. Can Vet J. 1984;25:435–9.
Weijer K, Hart AAM. Prognostic factors in feline mammary carcinoma. J Natl Cancer Inst. 1983;70:709–15.
Carpenter JL, Andrews LK, Holzworth J. Tumors and tumor-like lesions. In: Holzworth J, editor. Diseases of the cat: Medicine and surgery. Philadelphia: WB Saunders; 1987. p. 406–596.
Ogilvie GK. Feline mammary neoplasia. The compendium collection feline medicine and surgery in practice. Trenton: Veterinary Learning Systems; 1992. p. 74–81.
Crosby JH. The role of fine-needle aspiration biopsy in the diagnosis and management of palpable masses. J Med Assoc Ga. 1996;85:33–6.
Pontifex AH, Roberts FJ. Fine needle aspiration biopsy cytology in the diagnosis of inflammatory lesions. Acta Cytol. 1985;29:979–82.
Couto SS, Griffey SM, Duarte PC, Madewell BR. Feline vaccine-associated fibrosarcoma: morphologic distinctions. Vet Pathol. 2002;39:33–41.
Cameron AM, Faulkin Jr LJ. Hyperplastic and inflammatory. nodules in the canine mammary gland. J Natl Cancer Inst. 1971;47:1277–87.
Weijer K, Head KW, Misdorp W, Hampe JF. Feline malignant mammary tumors. I. Morphology and biology: some comparisons with human and canine mammary. J Natl Cancer Inst. 1972;49:1697–704.
Zappulli V, De Zan G, Cardazzo B, Bargelloni L, Castagnaro M. Feline mammary tumors in comparative oncology. J Dairy Res. 2005;72:98–106.
Misdorp W. Tumors of the mammary gland. In: Meuten DJ, editor. Tumors in domestic animals. 4th ed. Iowa: Blackwell; 2002. p. 575–606.
MacEwen EG. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990;9:125–36.
Liberman L, Bonaccio E, Hamele-Bena D, Abramson AF, Cohen MA, Dershaw DD. Benign and malignant phyllodes tumors: mammographic and sonographic findings. Radiology. 1996;198:121–4.
Schwarz RJ, Shrestha R. Needle aspiration of breast abscesses. Am J Surg. 2001;182:117–9.
Hansen PB, Axelsson CK. Treatment of breast abscess. An analysis of patient material and implementation of recommendations. Ugeskr Laeger. 2003;165:128–31.
Osterman K, Rahm V-A. Lactation mastitis: bacterial cultivation of breast milk, symptoms, treatment and outcome. J Hum Lact. 2000;16:297–302.
Kvist LJ, Rydhstroem H. Factors related to breast abscess after delivery: a population-based study. BJOG: Int J Obstet Gynaecol. 2005;112:1070–4.
Amir LH, Lumley J, Garland S. A failed RCT to determine if antibiotics prevent mastitis: cracked nipples colonized with staphylococcus aureus: a randomised treatment trial. BMC Pregnancy Childbirth. 2004;4:19.
Javanbakht J, Pedram B, Taheriyan MR, Khadivar F, Hosseini SH, Abdi FS, et al. Canine transmissible venereal tumor and seminoma: a cytohistopathology and chemotherapy study of tumors in the growth phase and during regression after chemotherapy. Tumour Biol. 2014;35(6):5493–500.
Acknowledgments
The authors thank Solmaz and Mahdieh Javanbakht for their help with this manuscript.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s13277-016-5472-5.
This article has been retracted at the request of the Editor-in-Chief, the International Society of Oncology and BioMarkers (ISOBM) and the Publisher per the Committee on Publication Ethics guidelines. The article shows evidence of irregularities in authorship during the submission process, there is strong reason to believe that the peer review process was compromised and the following figure has been duplicated:
Fig 7 "Photomicrography of fine needle cytology from malignant mammary tumor in a cat; variation in cell (anisocytosis) and nuclear (anisokaryosis) size are present,May-Grunwald-Giemsa stainingmethod, x 1,000" is duplicating Fig 1B "Coarse chromatin structures in all samples and lightly basophilic cytoplasm were associated with tumor cells, and these cells were round or ovoid, containing single, large, round nuclei of variable size, coarse nuclear chromatin, and single, prominent, centrally placed nucleoli also cell's nucleus, and nuclei were clear." published in: J. Javanbakht, B. Pedram, M. R. Taheriyan, F. Khadivar, S. H. Hosseini, F. S. Abdi, E. Hosseini, M. Moloudizargari, S H. Aghajanshakeri, S. Javaherypour, R. Shafiee, R. Emrani Bidi, Canine transmissible venereal tumor and seminoma: a cytohistopathology and chemotherapy study of tumors in the growth phase and during regression after chemotherapy, Tumor Biol. 2014; 35:6 5493-5500 DOI: 10.1007/s13277-014-1723-5
As such the validity of the content of this article cannot be verified.
About this article
Cite this article
Manesh, J.Y.Y., Shafiee, R., Pedram, B. et al. RETRACTED ARTICLE: Improving the diagnosis, treatment, and biology patterns of feline mammary intraepithelial lesions: a potential model for human breast masses with evidence from epidemiologic and cytohistopathologic studies. Tumor Biol. 35, 12109–12117 (2014). https://doi.org/10.1007/s13277-014-2515-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2515-7