Abstract
Background
TBC1 domain-containing kinase (TBCK) protein functions as a growth suppressor in certain cell types and as a tumor promoter in others. Although TBCK knockdown increases the responsiveness of cancer cells to anticancer drugs, the detailed mechanisms by which TBCK knockdown increases susceptibility to anticancer drugs remain unknown.
Objective
This study analyzed the role of TBCK in sensitivities to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and doxorubicin in human renal cancer cells.
Methods
Flow cytometry was employed to evaluate the extent of apoptosis. Western blotting, transient transfection, and lentiviral infection techniques were conducted to investigate the impact of TBCK on apoptosis-related protein expression and mitogen-activated protein kinase (MAPK).
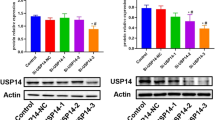
Results
TBCK knockdown in renal cancer cells inhibits ERK and Akt signaling pathways and increases TRAIL and doxorubicin sensitivity. In TBCK-knockdown Caki-1 cells, ERK and Akt phosphorylation was suppressed compared to control cell lines, and TRAIL and doxorubicin sensitivities were increased in these cells. In addition, the phosphorylation of PDK1 was suppressed in TBCK-suppressed cells, indicating that TBCK may be involved in the PDK1 and Akt signaling pathways. The introduction of dominantly active Akt into TBCK-suppressed cells restored their sensitivity to TRAIL. In addition, TBCK downregulation enhanced TRAIL sensitivity in different renal cancer cell lines.
Conclusions
These data suggest that TBCK could potentially have a crucial function in influencing the effects of anti-cancer drugs including TRAIL by modulating the signaling pathway involving Akt and PDK1 in human renal cancer cells.







Similar content being viewed by others
References
Beck-Wödl S, Harzer K, Sturm M, Buchert R, Rieß O, Mennel HD, Latta E, Pagenstecher A, Keber U (2018) Homozygous TBC1 domain-containing kinase (TBCK) mutation causes a novel lysosomal storage disease - a new type of neuronal ceroid lipofuscinosis (CLN15)? Acta Neuropathol Commun 6:145
Bordo D, Bork P (2002) The rhodanese/Cdc25 phosphatase superfamily. Sequence-structure-function relations. EMBO Rep 3:741–746
Itoh T, Satoh M, Kanno E, Fukuda M (2006) Screening for target rabs of TBC (Tre-2/Bub2/Cdc16) domain-containing proteins based on their rab-binding activity. Genes Cells 11:1023–1037
Kim YH, Lee YJ (2007) TRAIL apoptosis is enhanced by quercetin through akt dephosphorylation. J Cell Biochem 100:998–1009
Kim EA, Jang JH, Sung EG, Song IH, Kim JY, Lee TJ (2019) MiR-1208 increases the sensitivity to Cisplatin by Targeting TBCK in Renal Cancer cells. Int J Mol Sci 20
Komurov K, Padron D, Cheng T, Roth M, Rosenblatt KP, White MA (2010) Comprehensive mapping of the human kinome to epidermal growth factor receptor signaling. J Biol Chem 285:21134–21142
Liu Y, Yan X, Zhou T (2013) TBCK influences cell proliferation, cell size, and mTOR signaling pathway. PLoS ONE 8:e71349
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298:1912–1934
Mérino D, Lalaoui N, Morizot A, Solary E, Micheau O (2007) TRAIL in cancer therapy: present and future challenges. Expert Opin Ther Targets 11:1299–1314
Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis A, Tsoukalas D et al (2018) The akt pathway in oncology therapy and beyond (review). Int J Oncol 53:2319–2331
Ortiz-González XR, Tintos-Hernández JA, Keller K, Li X, Foley AR, Bharucha-Goebel DX, Kessler SK, Yum SW, Crino PB, He M et al (2018) Homozygous boricua TBCK mutation causes neurodegeneration and aberrant autophagy. Ann Neurol 83:153–165
Pan X, Eathiraj S, Munson M, Lambright DG (2006) TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature 442:303–306
Puduvalli VK, Sampath D, Bruner JM, Nangia J, Xu R, Kyritsis AP (2005) TRAIL-induced apoptosis in gliomas is enhanced by akt-inhibition and is independent of JNK activation. Apoptosis 10:233–243
Testa JR, Bellacosa A (2001) AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U S A 98:10983–10985
von Karstedt S, Montinaro A, Walczak H (2017) Exploring the TRAILs less traveled: TRAIL in cancer biology and therapy. Nat Rev Cancer 17:352–366
Wang F, Lin J, Xu R (2014) The molecular mechanisms of TRAIL resistance in cancer cells: help in designing new drugs. Curr Pharm Des 20:6714–6722
Wu J, Lu G (2021) Multiple functions of TBCK protein in neurodevelopment disorders and tumors. Oncol Lett 21:17
Wu J, Li Q, Li Y, Lin J, Yang D, Zhu G, Wang L, He D, Lu G, Zeng C (2014) A long type of TBCK is a novel cytoplasmic and mitotic apparatus-associated protein likely suppressing cell proliferation. J Genet Genomics 41:69–72
Yu L, Wei J, Liu P (2022) Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin Cancer Biol 85:69–94
Funding
This work was supported by the Basic Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2020R1F1A1048259).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Not applicable.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, CY., Jang, JH., Song, IH. et al. Suppression of TBCK enhances TRAIL-mediated apoptosis by causing the inactivation of the akt signaling pathway in human renal carcinoma Caki-1 cells. Genes Genom 45, 1357–1365 (2023). https://doi.org/10.1007/s13258-023-01453-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-023-01453-y