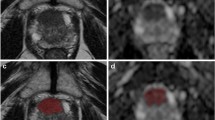
Abstract
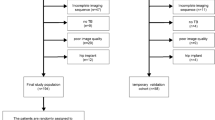
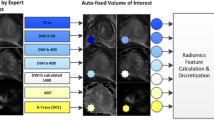
The purpose of this study was to develop Bi-parametric Magnetic Resonance Imaging (BP-MRI) based radiomics models for differentiation between benign and malignant prostate lesions, and to cross-vendor validate the generalization ability of the models. The prebiopsy BP-MRI data (T2-Weighted Image [T2WI] and the Apparent Diffusion Coefficient [ADC]) of 459 patients with clinical suspicion of prostate cancer were acquired using two scanners from different vendors. The prostate biopsies are the reference standard for diagnosing benign and malignant prostate lesions. The training set was 168 patients’ data from Siemens (Vendor 1), and the inner test set was 70 patients’ data from the same vendor. The external test set was 221 patients’ data from GE (Vendor 2). The lesion Region of Interest (ROI) was manually delineated by experienced radiologists. A total of 851 radiomics features including shape, first-order statistical, texture, and wavelet features were extracted from ROI in T2WI and ADC, respectively. Two feature-ranking methods (Minimum Redundancy Maximum Relevance [MRMR] and Wilcoxon Rank-Sum Test [WRST]) and three classifiers (Random Forest [RF], Support Vector Machine [SVM], and the Least Absolute Shrinkage and Selection Operator [LASSO] regression) were investigated for their efficacy in building single-parametric radiomics signatures. A biparametric radiomics model was built by combining the optimal single-parametric radiomics signatures. A comprehensive diagnosis model was built by combining the biparametric radiomics model with age and Prostate Specific Antigen (PSA) value using multivariable logistic regression. All models were built in the training set and independently validated in the inner and external test sets, and the performances of models in the diagnosis of benign and malignant prostate lesions were quantified by the Area Under the Receiver Operating Characteristic Curve (AUC). The mean AUCs of the inner and external test sets were calculated for each model. The non-inferiority test was used to test if the AUC of model in external test was not inferior to the AUC of model in inner test. Combining MRMR and LASSO produced the best-performing single-parametric radiomics signatures with the highest mean AUC of 0.673 for T2WI (inner test AUC = 0.729 vs. external test AUC = 0.616, p = 0.569) and the highest mean AUC of 0.810 for ADC (inner test AUC = 0.822 vs. external test AUC = 0.797, p = 0.102). The biparametric radiomics model produced a mean AUC of 0.833 (inner test AUC = 0.867 vs. external test AUC = 0.798, p = 0.051). The comprehensive diagnosis model had an improved mean AUC of 0.911 (inner test AUC = 0.935 vs. external test AUC = 0.886, p = 0.010). The comprehensive diagnosis model for differentiating benign from malignant prostate lesions was accurate and generalizable.



Similar content being viewed by others
References
Carroll PR, Parsons JK, Andriole G et al (2016) NCCN guidelines insights: prostate cancer early detection, version 2.2016. J Natl Compr Canc Netw 14:509–519. https://doi.org/10.6004/jnccn.2016.0060
Islami F, Moreira DM, Boffetta P, Freedland SJ (2014) A systematic review and meta-analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies. Eur Urol 66:1054. https://doi.org/10.1016/j.eururo.2014.08.059
Attard G, Antonarakis ES (2016) Prostate cancer: AR aberrations and resistance to abiraterone or enzalutamide. Nat Rev Urol 13:697–698. https://doi.org/10.1038/nrurol.2016.212
Ploussard G, Nicolaiew N, Marchand C et al (2014) Prospective evaluation of an extended 21-core biopsy scheme as initial prostate cancer diagnostic strategy. Eur Urol 65:154–161. https://doi.org/10.1016/j.eururo.2012.05.049
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent—update 2013. Eur Urol 65:124–137. https://doi.org/10.1016/j.eururo.2013.09.046
Tricoli JV (2004) Detection of prostate cancer and predicting progression: current and future diagnostic markers. Clin Cancer Res 10:3943–3953. https://doi.org/10.1158/1078-0432.CCR-03-0200
Schieda N, Coffey N, Gulavita P et al (2014) Prostatic ductal adenocarcinoma: an aggressive tumour variant unrecognized on T2 weighted magnetic resonance imaging (MRI). Eur Radiol 24:1349–1356. https://doi.org/10.1007/s00330-014-3150-9
Tamada T, Sone T, Jo Y et al (2014) Diffusion-weighted MRI and its role in prostate cancer. NMR Biomed 27:25–38. https://doi.org/10.1002/nbm.2956
Johnson DC, Raman SS, Mirak SA et al (2019) Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol 75:712–720. https://doi.org/10.1016/j.eururo.2018.11.031
Cameron A, Khalvati F (2015) MAPS: a quantitative radiomics approach for prostate cancer detection. IEEE Trans Biomed Eng 63:12
Xu M, Fang M, Zou J et al (2019) Using biparametric MRI radiomics signature to differentiate between benign and malignant prostate lesions. Eur J Radiol 114:38–44. https://doi.org/10.1016/j.ejrad.2019.02.032
Liu B, Cheng J, Guo DJ et al (2019) Prediction of prostate cancer aggressiveness with a combination of radiomics and machine learning-based analysis of dynamic contrast-enhanced MRI. Clin Radiol 74:896.e1-896.e8. https://doi.org/10.1016/j.crad.2019.07.011
Chen T, Li M, Gu Y et al (2019) Prostate cancer differentiation and aggressiveness: assessment with a radiomic-based model vs. PI-RADS v2. J Magn Reson Imaging 49:875–884. https://doi.org/10.1002/jmri.26243
Tong C (2006) Refinement strategies for stratified sampling methods. Reliab Eng Syst Saf 91:1257–1265. https://doi.org/10.1016/j.ress.2005.11.027
Maes F, Collignon A, Vandermeulen D et al (1997) Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 16:187–198. https://doi.org/10.1109/42.563664
El-Gamal FE-ZA, Elmogy M, Atwan A (2016) Current trends in medical image registration and fusion. Egypt Inform J 17:99–124. https://doi.org/10.1016/j.eij.2015.09.002
van Griethuysen JJ, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Can Res 77:e104. https://doi.org/10.1158/0008-5472.CAN-17-0339
Jian J, Li Y, Pickhardt PJ et al (2021) MR image-based radiomics to differentiate type Ι and type ΙΙ epithelial ovarian cancers. Eur Radiol 31:403–410. https://doi.org/10.1007/s00330-020-07091-2
Li Y, Jian J, Pickhardt PJ et al (2020) MRI-based machine learning for differentiating borderline from malignant epithelial ovarian tumors: a multicenter study. J Magn Reson Imaging 52:897–904. https://doi.org/10.1002/jmri.27084
Dong X, Dan X, Yawen A et al (2020) Identifying sarcopenia in advanced non-small cell lung cancer patients using skeletal muscle CT radiomics and machine learning. Thorac Cancer 11:2650–2659. https://doi.org/10.1111/1759-7714.13598
Germanese D, Mercatelli L, Colantonio S (2019) Radiomics to predict prostate cancer aggressiveness: a preliminary study. In: 2019 IEEE 19th International Conference on Bioinformatics and Bioengineering (BIBE). pp 972–976. https://doi.org/10.1109/BIBE.2019.00181
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577. https://doi.org/10.1148/radiol.2015151169
Parmar C, Grossmann P, Bussink J et al (2015) Machine learning methods for quantitative radiomic biomarkers. Sci Rep 5:13087. https://doi.org/10.1038/srep13087
Peng H, Long F, Ding C (2005) Feature selection based on mutual information: criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans Pattern Anal Mach Intell 27:1226–1238. https://doi.org/10.1109/TPAMI.2005.159
Zhao W, Xu Y, Yang Z et al (2019) Development and validation of a radiomics nomogram for identifying invasiveness of pulmonary adenocarcinomas appearing as subcentimeter ground-glass opacity nodules. Eur J Radiol 112:161–168. https://doi.org/10.1016/j.ejrad.2019.01.021
Fernández-Delgado M, Cernadas E, Barro S, Amorim D (2014) Do we need hundreds of classifiers to solve real world classification problems? J Mach Learn Res 15:3133–3181
Parmar C, Grossmann P, Rietveld D et al (2015) Radiomic machine-learning classifiers for prognostic biomarkers of head and neck cancer. Front Oncol 5:272. https://doi.org/10.3389/fonc.2015.00272
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36. https://doi.org/10.1148/radiology.143.1.7063747
Jin H, Lu Y (2009) A non-inferiority test of areas under two parametric ROC curves. Contemp Clin Trials 30:375–379. https://doi.org/10.1016/j.cct.2009.03.003
Salazar AJ, Romero JA, Bernal OA et al (2016) Noninferiority and equivalence evaluation of clinical performance among computed radiography, film, and digitized film for telemammography services. Int J Telemed Appl. https://doi.org/10.1155/2016/3642960
Vollmar C, O’Muircheartaigh J, Barker GJ et al (2010) Identical, but not the same: intra-site and inter-site reproducibility of fractional anisotropy measures on two 3.0T scanners. Neuroimage 51:1384–1394. https://doi.org/10.1016/j.neuroimage.2010.03.046
Helmer KG, Chou M-C, Preciado RI et al (2016) Multi-site study of diffusion metric variability: effects of site, vendor, field strength, and echo time on regions-of-interest and histogram-bin analyses. Proc SPIE Int Soc Opt Eng. https://doi.org/10.1117/12.2217445
Woźnicki P, Westhoff N, Huber T et al (2020) Multiparametric MRI for prostate cancer characterization: combined use of radiomics model with PI-RADS and clinical parameters. Cancers (Basel). https://doi.org/10.3390/cancers12071767
Liang L, Zhi X, Sun Y et al (2021) A nomogram based on a multiparametric ultrasound radiomics model for discrimination between malignant and benign prostate lesions. Front Oncol 11:610785. https://doi.org/10.3389/fonc.2021.610785
Hansen NL, Koo BC, Warren AY et al (2017) Sub-differentiating equivocal PI-RADS-3 lesions in multiparametric magnetic resonance imaging of the prostate to improve cancer detection. Eur J Radiol 95:307–313. https://doi.org/10.1016/j.ejrad.2017.08.017
Chatterjee A, Watson G, Myint E et al (2015) Changes in epithelium, stroma, and lumen space correlate more strongly with gleason pattern and are stronger predictors of prostate ADC changes than cellularity metrics. Radiology 277:751–762. https://doi.org/10.1148/radiol.2015142414
Pierre T, Cornud F, Colléter L, Beuvon F, Foissac F (2018) Diffusion-weighted imaging of the prostate: should we use quantitative metrics to better characterize focal lesions originating in the peripheral zone? Eur Radiol 28:2236–2245. https://doi.org/10.1007/s00330-017-5107-2
Vallières M, Freeman CR, Skamene SR, El Naqa I (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 60:5471–5496. https://doi.org/10.1088/0031-9155/60/14/5471
Coroller TP, Grossmann P, Hou Y et al (2015) CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol 114:345. https://doi.org/10.1016/j.radonc.2015.02.015
Saeys Y, Inza I, Larrañaga P (2007) A review of feature selection techniques in bioinformatics. Bioinformatics 23:2507–2517. https://doi.org/10.1093/bioinformatics/btm344
Liang C, Huang Y, He L, Chen X, Ma Z, Dong D, Tian J, Liang C, Liu Z (2016) The development and validation of a CT-based radiomics signature for the preoperative discrimination of stage I-II and stage III-IV colorectal cancer. Oncotarget 21:31401–31412
Singanamalli A, Rusu M, Sparks RE et al (2016) Identifying in vivo DCE MRI markers associated with microvessel architecture and gleason grades of prostate cancer. J Magn Reson Imaging 43:149. https://doi.org/10.1002/jmri.24975
Huang Y, Liu Z, He L et al (2016) Radiomics signature: a potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology 281:947–957. https://doi.org/10.1148/radiol.2016152234
Castiglioni I, Gilardi MC (2018) Radiomics: is it time to compose the puzzle? Clin Transl Imaging 6:411–413. https://doi.org/10.1007/s40336-018-0302-y
Shrestha S, Alsadoon A, Prasad PWC et al (2021) A novel solution of using deep learning for prostate cancer segmentation: enhanced batch normalization. Multimed Tools Appl. https://doi.org/10.1007/s11042-021-10779-2
Tomaszewski MR, Gillies RJ (2021) The biological meaning of radiomic features. Radiology. https://doi.org/10.1148/radiol.2021202553
Funding
This work was supported by National Natural Science Foundation of China [Grant Numbers: 61801474, 61801475]; CAS-VPST Slik Road Science Fund 2018 [Grant Number: GJHZ1857]; Science and Technology Plan Project of Tianjin [grant number: 19YDYGHZ00030]; Suzhou science and technology plan project [Grant Number: SYG201908, SS201863].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Ethical approval
Institutional Review Board approval was obtained in concordance with the standards of the First Affiliated Hospital of Soochow University Ethics Committee (2020; approval no. 064). All procedures performed in this study, which involved human participants, were in accordance with the ethical standards of the institutional and/or nation research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Approved by the ethics committee of the First Affiliated Hospital of Soochow University Ethics Committee, this retrospective analysis was involving MRI of patients in and informed consent was waived for all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ji, X., Zhang, J., Shi, W. et al. Bi-parametric magnetic resonance imaging based radiomics for the identification of benign and malignant prostate lesions: cross-vendor validation. Phys Eng Sci Med 44, 745–754 (2021). https://doi.org/10.1007/s13246-021-01022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-021-01022-1