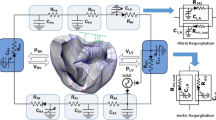
Abstract
Background
In object-oriented or acausal modelling, components of the model can be connected topologically, following the inherent structure of the physical system, and system equations can be formulated automatically. This technique allows individuals without a mathematics background to develop knowledge-based models and facilitates collaboration in multidisciplinary fields like biomedical engineering. This study conducts a preclinical evaluation of a ventricular assist device (VAD) in assisting advanced-stage heart failure patients in an acausal modelling environment.
Methods
A comprehensive object-oriented model of the cardiovascular system with a VAD is developed in MATLAB/SIMSCAPE, and its hemodynamic behaviour is studied. An analytically derived pump model is calibrated for the experimental prototype of the Istanbul Heart VAD. Hemodynamics are produced under healthy, diseased, and assisted conditions. The study features a comprehensive collection of advanced-stage heart failure patients’ data from the literature to identify parameters for disease modelling and to validate the resulting hemodynamics.
Results
Regurgitation, suction, and optimal speeds are identified, and trends in different hemodynamic parameters are observed for the simulated pathophysiological conditions. Using pertinent parameters in disease modelling allows for more accurate results compared to the traditional approach of arbitrary reduction in left ventricular contractility to model dilated cardiomyopathy.
Conclusion
The current research provides a comprehensive and validated framework for the preclinical evaluation of cardiac assist devices. Due to its object-oriented nature, the featured model is readily modifiable for other cardiovascular diseases for studying the effect of pump operating conditions on hemodynamics and vice versa in silico and hybrid mock circulatory loops. The work also provides a potential teaching tool for understanding the pathophysiology of heart failure, diagnosis rationale, and degree of assist requirements.








Similar content being viewed by others
References
Hakman, M., and T. Groth. Object-oriented biomedical system modeling—the rationale. Comput. Methods Programs Biomed. 59(1):1–17, 1999. https://doi.org/10.1016/s0169-2607(98)00097-2.
Kofránek, J., M. Mateják, P. Privitzer, and M. Tribula. Causal or acausal modeling: labour for humans or labour for machines. In: Technical Computing Prague, pp. 1–16, 2008.
Shi, Y., P. Lawford, and R. Hose. Review of zero-D and 1-D models of blood flow in the cardiovascular system. BioMed. Eng. OnLine. 10(1):33, 2011. https://doi.org/10.1186/1475-925X-10-33.
Son, J., D. Du, and Y. Du. Modelling and control of a failing heart managed by a left ventricular assist device. Biocybern. Biomed. Eng. 40(1):559–573, 2020. https://doi.org/10.1016/j.bbe.2020.01.014.
Seongjin, C., J. E. Antaki, R. Boston, and D. Thomas. A sensorless approach to control of a turbodynamic left ventricular assist system. IEEE Trans. Control Syst. Technol. 9(3):473–482, 2001. https://doi.org/10.1109/87.918900.
Faragallah, G., Y. Wang, E. Divo, and M. Simaan. A new control system for left ventricular assist devices based on patient-specific physiological demand. Inverse Probl. Sci. Eng. 20(5):721–734, 2012. https://doi.org/10.1080/17415977.2012.667092.
Yuan, V., A. Verma, N. K. Schiavone, D. N. Rosenthal, and A. L. Marsden. A mechanistic lumped parameter model of the Berlin Heart EXCOR to analyze device performance and physiologic interactions. Cardiovasc. Eng. Technol. 2022. https://doi.org/10.1007/s13239-021-00603-1.
Granegger, M., H. Dave, W. Knirsch, B. Thamsen, M. Schweiger, and M. Hübler. A valveless pulsatile pump for the treatment of heart failure with preserved ejection fraction: a simulation study. Cardiovasc. Eng. Technol. 10(1):69–79, 2019. https://doi.org/10.1007/s13239-018-00398-8.
Prather, R., E. Divo, A. Kassab, and W. DeCampli. In silico analysis of outflow graft implantation orientation and cerebral thromboembolism incidence for full LVAD support. Comput. Methods Biomech. Biomed. Eng. 25(11):1249–1261, 2022. https://doi.org/10.1080/10255842.2021.2005789.
de Canete, J. F., P. D. Saz-Orozco, D. Moreno-Boza, and E. Duran-Venegas. Object-oriented modeling and simulation of the closed loop cardiovascular system by using SIMSCAPE. Comput. Biol. Med. 43(4):323–333, 2013. https://doi.org/10.1016/j.compbiomed.2013.01.007.
Rosalia, L., C. Ozturk, D. Van Story, M. A. Horvath, and E. T. Roche. Object-oriented lumped-parameter modeling of the cardiovascular system for physiological and pathophysiological conditions. Adv. Theory Simul. 4(3):2000216, 2021. https://doi.org/10.1002/adts.202000216.
Rosalia, L., C. Ozturk, and E. T. Roche. Lumped-parameter and finite element modeling of heart failure with preserved ejection fraction. J. Vis. Exp. 2021. https://doi.org/10.3791/62167.
Rosalia, L., C. Ozturk, J. Coll-Font, Y. Fan, Y. Nagata, M. Singh, D. Goswami, A. Mauskapf, S. Chen, R. A. Eder, E. M. Goffer, J. H. Kim, S. Yurista, B. P. Bonner, A. N. Foster, R. A. Levine, E. R. Edelman, M. Panagia, J. L. Guerrero, E. T. Roche, and C. T. Nguyen. A soft robotic sleeve mimicking the haemodynamics and biomechanics of left ventricular pressure overload and aortic stenosis. Nat. Biomed. Eng. 6(10):1134–1147, 2022. https://doi.org/10.1038/s41551-022-00937-8.
Ozturk, C., L. Rosalia, and E. T. Roche. A multi-domain simulation study of a pulsatile-flow pump device for heart failure with preserved ejection fraction. Front. Physiol. 2022. https://doi.org/10.3389/fphys.2022.815787.
Shi, Y., and T. Korakianitis. Numerical simulation of cardiovascular dynamics with left heart failure and in-series pulsatile ventricular assist device. Artif. Organs. 30(12):929–948, 2006. https://doi.org/10.1111/j.1525-1594.2006.00326.x.
Shi, Y., and T. Korakianitis. Impeller-pump model derived from conservation laws applied to the simulation of the cardiovascular system coupled to heart-assist pumps. Comput. Biol. Med. 93:127–138, 2018. https://doi.org/10.1016/j.compbiomed.2017.12.012.
Klabunde, R. Cardiovascular Physiology Concepts. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2011.
Bozkurt, S., W. Paracha, K. Bakaya, and S. Schievano. Patient-specific modelling and parameter optimisation to simulate dilated cardiomyopathy in children. Cardiovasc. Eng. Technol. 2022. https://doi.org/10.1007/s13239-022-00611-9.
Avanzolini, G., P. Barbini, A. Cappello, and G. Cevenini. CADCS simulation of the closed-loop cardiovascular system. Int. J. Biomed. Comput. 22(1):39–49, 1988. https://doi.org/10.1016/0020-7101(88)90006-2.
Sagawa, K. Cardiac Contraction and the Pressure-Volume Relationship. Oxford: Oxford University Press, 1988.
Campbell, K. B., J. A. Ringo, Y. Wakao, P. A. Klavano, and J. E. Alexander. Internal capacitance and resistance allow prediction of right ventricle outflow. Am. J. Physiol. 243(1):H99–H112, 1982. https://doi.org/10.1152/ajpheart.1982.243.1.H99.
Capoccia, M. Review of mathematical modelling of the interactions between left ventricular assist devices and the cardiovascular system with a simulation based approach as a tool for training and preoperative planning. In: Cardiovascular and Pulmonary Artificial Organs: Educational Training Simulators, edited by C. De Lazzari, and M. Pirtskhalava. Roma: CNR Edizioni, 2017.
Schima, H., J. Honigschnabel, W. Trubel, and H. Thoma. Computer simulation of the circulatory system during support with a rotary blood pump. ASAIO Trans. 36(3):M252–M254, 1990.
Ozturk, C., I. B. Aka, and I. Lazoglu. Effect of blade curvature on the hemolytic and hydraulic characteristics of a centrifugal blood pump. Int. J. Artif. Organs. 41(11):730–737, 2018. https://doi.org/10.1177/0391398818785558.
Lazoglu, I., D. S. Kucukaksu, C. Ozturk, I. B. Aka, V. Bakuy, N. Arat, O. Yalcin, E. Ugurel, P. Celikbilek Erkasap, E. Aksoy, and S. Ruacan. A short-term in vivo evaluation of the Istanbul Heart Left Ventricular Assist Device in a pig model. Exp. Clin. Transplant. 2019. https://doi.org/10.6002/ect.2019.0110.
Choi, S. Modeling and Control of Left Ventricular Assist System. Pittsburgh: University of Pittsburgh, 1998.
Comunale, G., P. Peruzzo, B. Castaldi, R. Razzolini, G. Di Salvo, M. A. Padalino, and F. M. Susin. Understanding and recognition of the right ventricular function and dysfunction via a numerical study. Sci. Rep. 11(1):3709, 2021. https://doi.org/10.1038/s41598-021-82567-9.
Klingensmith, M. E. The Washington Manual of Surgery. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008. (in English)
Barnea, O., and N. Sheffer. A computer model for analysis of fluid resuscitation. Comput. Biol. Med. 23(6):443–454, 1993. https://doi.org/10.1016/0010-4825(93)90092-f.
Nauta, J. F., Y. M. Hummel, J. Tromp, W. Ouwerkerk, P. van der Meer, X. Jin, C. S. P. Lam, J. J. Bax, M. Metra, N. J. Samani, P. Ponikowski, K. Dickstein, S. D. Anker, C. C. Lang, L. L. Ng, F. Zannad, G. S. Filippatos, D. J. van Veldhuisen, J. P. van Melle, and A. A. Voors. Concentric vs. eccentric remodelling in heart failure with reduced ejection fraction: clinical characteristics, pathophysiology and response to treatment. Eur. J. Heart Fail. 22(7):1147–1155, 2020. https://doi.org/10.1002/ejhf.1632.
Pak, P. H., W. L. Maughan, K. L. Baughman, and D. A. Kass. Marked discordance between dynamic and passive diastolic pressure–volume relations in idiopathic hypertrophic cardiomyopathy. Circulation. 94(1):52–60, 1996. https://doi.org/10.1161/01.CIR.94.1.52.
O’Neill, B. Some useful moment results in sampling problems. Am. Stat. 68(4):282–296, 2014. https://doi.org/10.1080/00031305.2014.966589.
Ishihara, H., M. Yokota, T. Sobue, and H. Saito. Relation between ventriculoarterial coupling and myocardial energetics in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 23(2):406–416, 1994. https://doi.org/10.1016/0735-1097(94)90428-6.
Senzaki, H., B. Fetics, C.-H. Chen, and D. A. Kass. Comparison of ventricular pressure relaxation assessments in human heart failure: quantitative influence on load and drug sensitivity analysis. J. Am. Coll. Cardiol. 34(5):1529–1536, 1999.
Warriner, D., A. Brown, S. Varma, P. Sheridan, P. Lawford, D. Hose, A. Al-Mohammad, and Y. Shi. Closing the loop: modelling of heart failure progression from health to end-stage using a meta-analysis of left ventricular pressure–volume loops. PLoS ONE. 9:e114153, 2014. https://doi.org/10.1371/journal.pone.0114153.
Carroll, J. D., S. Shroff, P. Wirth, M. Halsted, and S. I. Rajfer. Arterial mechanical properties in dilated cardiomyopathy. Aging and the response to nitroprusside. J. Clin. Investig. 87(3):1002–1009, 1991. https://doi.org/10.1172/jci115058.
Stevenson, L. W., D. Bellil, M. Grover-Mckay, R. C. Brunken, M. Schwaiger, J. H. Tillisch, and H. R. Schelbert. Effects of afterload reduction (diuretics and vasodilators) on left ventricular volume and mitral regurgitation in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 60(8):654–658, 1987. https://doi.org/10.1016/0002-9149(87)90376-6.
Ribner, H. S., D. A. Plucinski, A. M. Hsieh, D. Bresnahan, A. Molteni, J. Askenazi, and M. Lesch. Acute effects of digoxin on total systemic vascular resistance in congestive heart failure due to dilated cardiomyopathy: a hemodynamic-hormonal study. Am. J. Cardiol. 56(13):896–904, 1985. https://doi.org/10.1016/0002-9149(85)90778-7.
Merillon, J. P., G. Fontenier, J. F. Lerallut, M. Y. Jaffrin, J. Chastre, P. Assayag, G. Motte, and R. Gourgon. Aortic input impedance in heart failure: comparison with normal subjects and its changes during vasodilator therapy. Eur. Heart J. 5(6):447–455, 1984. https://doi.org/10.1093/oxfordjournals.eurheartj.a061690.
Bombardini, T., M. F. Costantino, R. Sicari, Q. Ciampi, L. Pratali, and E. Picano. End-systolic elastance and ventricular-arterial coupling reserve predict cardiac events in patients with negative stress echocardiography. BioMed. Res. Int. 2013:235194, 2013. https://doi.org/10.1155/2013/235194.
Laskey, W. K., H. G. Parker, V. A. Ferrari, W. G. Kussmaul, and A. Noordergraaf. Estimation of total systemic arterial compliance in humans. J. Appl. Physiol. (1985). 69(1):112–119, 1990. https://doi.org/10.1152/jappl.1990.69.1.112.
Cotter, G., Y. Moshkovitz, E. Kaluski, O. Milo, Y. Nobikov, A. Schneeweiss, R. Krakover, and Z. Vered. The role of cardiac power and systemic vascular resistance in the pathophysiology and diagnosis of patients with acute congestive heart failure. Eur. J. Heart Fail. 5(4):443–451, 2003. https://doi.org/10.1016/S1388-9842(03)00100-4.
Her, A. Y., J. Y. Kim, E. Y. Choi, S. A. Kim, R. S. Jae, C. Y. Shim, S. M. Kang, J. W. Ha, and N. Chung. Value of ventricular stiffness index and ventriculoarterial interaction in patients with nonischemic dilated cardiomyopathy. Circ. J. 73(9):1683–1690, 2009. https://doi.org/10.1253/circj.cj-09-0046.
Chemla, D., J. L. Hébert, C. Coirault, K. Zamani, I. Suard, P. Colin, and Y. Lecarpentier. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am. J. Physiol. 274(2):H500–H505, 1998. https://doi.org/10.1152/ajpheart.1998.274.2.H500.
Pellegrini, P., A. Rossi, M. Pasotti, C. Raineri, M. Cicoira, S. Bonapace, F. L. Dini, P. L. Temporelli, C. Vassanelli, R. Vanderpool, R. Naeije, and S. Ghio. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest. 145(5):1064–1070, 2014. https://doi.org/10.1378/chest.13-1510.
Son, J., D. Du, and Y. Du. Stochastic modeling and dynamic analysis of the cardiovascular system with rotary left ventricular assist devices. Math. Probl. Eng. 2019:7179317, 2019. https://doi.org/10.1155/2019/7179317.
Schreuder, J. J., F. H. van der Veen, E. T. van der Velde, F. Delahaye, O. Alfieri, O. Jegaden, R. Lorusso, J. R. C. Jansen, S. A. A. P. Hoeksel, G. Finet, M. Volterrani, H.-G. Kaulbach, J. Baan, and H. J. J. Wellens. Left ventricular pressure–volume relationships before and after cardiomyoplasty in patients with heart failure. Circulation. 96(9):2978–2986, 1997. https://doi.org/10.1161/01.CIR.96.9.2978.
Berk, F., S. Isgoren, H. Demir, G. Kozdag, D. Ural, and B. Komsuoglu. Evaluation of left ventricular function and volume in patients with dilated cardiomyopathy: gated myocardial single-photon emission tomography (SPECT) versus echocardiography. Ann. Saudi Med. 25(3):198–204, 2005. https://doi.org/10.5144/0256-4947.2005.198.
Suzuki, J.-I., G. R. Caputo, T. Masui, J.-M. Chang, M. O’Sullivan, and C. B. Higgins. Assessment of right ventricular diastolic and systolic function in patients with dilated cardiomyopathy using cine magnetic resonance imaging. Am. Heart J. 122(4, Part1):1035–1040, 1991. https://doi.org/10.1016/0002-8703(91)90469-X.
Bielecka-Dabrowa, A., S. von Haehling, W. S. Aronow, M. I. Ahmed, J. Rysz, and M. Banach. Heart failure biomarkers in patients with dilated cardiomyopathy. Int. J. Cardiol. 168(3):2404–2410, 2013. https://doi.org/10.1016/j.ijcard.2013.01.157.
D’Andrea, A., G. Salerno, R. Scarafile, L. Riegler, R. Gravino, F. Castaldo, R. Cocchia, G. Limongelli, M. Romano, P. Calabrò, G. Nigro, S. Cuomo, E. Bossone, P. Caso, and R. Calabrò. Right ventricular myocardial function in patients with either idiopathic or ischemic dilated cardiomyopathy without clinical sign of right heart failure: effects of cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 32(8):1017–1029, 2009. https://doi.org/10.1111/j.1540-8159.2009.02434.x.
Tachi, M., Y. Amano, K. Inui, M. Takeda, F. Yamada, K. Asai, and S. Kumita. Relationship of postcontrast myocardial T1 value and delayed enhancement to reduced cardiac function and serious arrhythmia in dilated cardiomyopathy with left ventricular ejection fraction less than 35. Acta Radiol. 57(4):430–436, 2016. https://doi.org/10.1177/0284185115580840.
Birks, E. J., S. G. Drakos, S. R. Patel, B. D. Lowes, C. H. Selzman, R. C. Starling, J. Trivedi, M. S. Slaughter, P. Alturi, D. Goldstein, S. Maybaum, J. Y. Um, K. B. Margulies, J. Stehlik, C. Cunningham, D. J. Farrar, and J. E. Rame. Prospective multicenter study of myocardial recovery using left ventricular assist devices [RESTAGE-HF (Remission from Stage D Heart Failure)]. Circulation. 142(21):2016–2028, 2020. https://doi.org/10.1161/CIRCULATIONAHA.120.046415.
Streitner, F., T. Herrmann, J. Kuschyk, S. Lang, C. Doesch, T. Papavassiliu, I. Streitner, C. Veltmann, D. Haghi, and M. Borggrefe. Impact of shocks on mortality in patients with ischemic or dilated cardiomyopathy and defibrillators implanted for primary prevention. PLoS ONE. 8(5):e63911, 2013. https://doi.org/10.1371/journal.pone.0063911.
Ortega, A., E. Tarazón, C. Gil-Cayuela, M. García-Manzanares, L. Martínez-Dolz, F. Lago, J. R. González-Juanatey, J. Cinca, E. Jorge, M. Portolés, E. Roselló-Lletí, and M. Rivera. Intercalated disc in failing hearts from patients with dilated cardiomyopathy: its role in the depressed left ventricular function. PLoS ONE. 12(9):e0185062, 2017. https://doi.org/10.1371/journal.pone.0185062.
Sato, H., M. Hori, H. Ozaki, H. Yokoyama, K. Imai, M. Morikawa, H. Takeda, M. Inoue, and T. Kamada. Exercise-induced upward shift of diastolic left ventricular pressure–volume relation in patients with dilated cardiomyopathy. Effects of beta-adrenoceptor blockade. Circulation. 88(5):2215–2223, 1993. https://doi.org/10.1161/01.CIR.88.5.2215.
Uriel, N., D. Burkhoff, J. D. Rich, S. G. Drakos, J. J. Teuteberg, T. Imamura, D. Rodgers, J. Raikhelkar, E. E. Vorovich, C. H. Selzman, G. Kim, and G. Sayer. Impact of hemodynamic ramp test-guided HVAD speed and medication adjustments on clinical outcomes. Circ. Heart Fail. 12(4):e006067, 2019. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006067.
Uriel, N., G. Sayer, K. Addetia, S. Fedson, G. H. Kim, D. Rodgers, E. Kruse, K. Collins, S. Adatya, N. Sarswat, U. P. Jorde, C. Juricek, T. Ota, V. Jeevanandam, D. Burkhoff, and R. M. Lang. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail. 4(3):208–217, 2016. https://doi.org/10.1016/j.jchf.2015.10.001.
Aune, E., M. Bækkevar, O. Rødevand, and J. E. Otterstad. Reference values for left ventricular volumes with real-time 3-dimensional echocardiography. Scand. Cardiovasc. J. 44(1):24–30, 2010. https://doi.org/10.3109/14017430903114446.
Hall, J. E., and M. E. Hall. Guyton and Hall Textbook of Medical Physiology e-Book. Amsterdam: Elsevier Health Sciences, 2020.
Goodman, J., and S. Lerakis. The aortic valve: the gatekeeper of the LVAD. CASE (Phila). 4(5):341–342, 2020. https://doi.org/10.1016/j.case.2020.05.015.
Hydren, J. R., J. R. Gifford, C. L. Jarrett, S. H. Park, K. L. Shields, R. M. Broxterman, A. C. Kithas, A. V. Bisconti, T. S. Thurston, S. M. Ratchford, D. W. Wray, J. Stehlik, C. H. Selzman, S. G. Drakos, and R. S. Richardson. Vascular function in continuous-flow left ventricular assist device recipients: effect of a single pulsatility treatment session. Am. J. Physiol. Regul. Integr. Comp. Physiol. 320(4):R425–R437, 2021. https://doi.org/10.1152/ajpregu.00274.2020.
Sujino, Y., K. Kuroda, K. Yoshitake, N. Yagi, E. Anegawa, H. Mochizuki, K. Iwasaki, S. Nakajima, T. Watanabe, M. Yanase, S. Fukushima, T. Fujita, J. Kobayashi, and N. Fukushima. Clinical potential of hemodynamic ramp test by simultaneous echocardiography and right heart catheterization for aortic insufficiency in a patient with continuous-flow left ventricular assist device. J. Artif. Organs. 24(2):265–268, 2021. https://doi.org/10.1007/s10047-020-01210-y.
Dunlay, S. M., S. J. Park, K. Chandrasekaran, J. O. Choi, N. L. Pereira, L. D. Joyce, R. C. Daly, J. M. Stulak, and S. S. Kushwaha. Changes in left ventricular ejection fraction following implantation of left ventricular assist device as destination therapy. J. Heart Lung Transplant. 32(4, Supplement):S116, 2013. https://doi.org/10.1016/j.healun.2013.01.244.
Travis, A. R., G. A. Giridharan, G. M. Pantalos, R. D. Dowling, S. D. Prabhu, M. S. Slaughter, M. Sobieski, A. Undar, D. J. Farrar, and S. C. Koenig. Vascular pulsatility in patients with a pulsatile- or continuous-flow ventricular assist device. J. Thorac. Cardiovasc. Surg. 133(2):517–524, 2007. https://doi.org/10.1016/j.jtcvs.2006.09.057.
Tzallas, A. T., N. S. Katertsidis, E. C. Karvounis, M. G. Tsipouras, G. Rigas, Y. Goletsis, K. Zielinski, L. Fresiello, A. D. Molfetta, G. Ferrari, J. V. Terrovitis, M. G. Trivella, and D. I. Fotiadis. Modeling and simulation of speed selection on left ventricular assist devices. Comput. Biol. Med. 51:128–139, 2014. https://doi.org/10.1016/j.compbiomed.2014.04.013.
Markham, D. W., and M. H. Drazner. Measuring nonpulsatile blood pressure. Circ. Heart Fail. 6(5):879–880, 2013. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000579.
Funding
This Research Project (318S143) was funded by the Scientific and Technological Research Council of Turkey (TUBITAK).
Author information
Authors and Affiliations
Contributions
KM proposed and conducted the research, drafted the manuscript, and conducted revisions. IL supervised the doctoral research and revised the manuscript. SK advised on the disease modelling and provided clinical insights for the research. All authors approved the submission of the paper.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Associate Editor Zhenglun Alan Wei oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehmood, K., Lazoglu, I. & Küçükaksu, D. Acausal Modelling of Advanced-Stage Heart Failure and the Istanbul Heart Ventricular Assist Device Support with Patient Data. Cardiovasc Eng Tech 14, 726–741 (2023). https://doi.org/10.1007/s13239-023-00683-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-023-00683-1