Abstract
Purpose
Worldwide, cardiovascular disease is the leading cause of hospitalization and death. Recently, the use of magnetizable nanoparticles for medical drug delivery has received much attention for potential treatment of both cancer and cardiovascular disease. However, proper understanding of the interacting magnetic field forces and the hydrodynamics of blood flow is needed for effective implementation. This paper presents the computational results of simulated implant assisted medical drug targeting (IA-MDT) via induced magnetism intended for administering patient specific doses of therapeutic agents to specific sites in the cardiovascular system. The drug delivery scheme presented in this paper functions via placement of a faintly magnetizable stent at a diseased location in the carotid artery, followed by delivery of magnetically susceptible drug carriers guided by the local magnetic field. Using this method, the magnetic stent can apply high localized magnetic field gradients within the diseased artery, while only exposing the neighboring tissues, arteries, and organs to a modest magnetic field. The localized field gradients also produce the forces needed to attract and hold drug-containing magnetic nanoparticles at the implant site for delivering therapeutic agents to treat in-stent restenosis.
Methods
The multi-physics computational model used in this work is from our previous work and has been slightly modified for the case scenario presented in this paper. The computational model is used to analyze pulsatile blood flow, particle motion, and particle capture efficiency in a magnetic stented region using the magnetic properties of magnetite (Fe3O4) and equations describing the magnetic forces acting on particles produced by an external cylindrical electromagnetic coil. The electromagnetic coil produces a uniform magnetic field in the computational arterial flow model domain, while both the particles and the implanted stent are paramagnetic. A Eulerian-Lagrangian technique is adopted to resolve the hemodynamic flow and the motion of particles under the influence of a range of magnetic field strengths (Br = 2T, 4T, 6T, and 8T). Particle diameter sizes of 10 nm–4 µm in diameter were evaluated. Two dimensionless numbers were evaluated in this work to characterize relative effects of Brownian motion (BM), magnetic force induced particle motion, and convective blood flow on particle motion.
Results
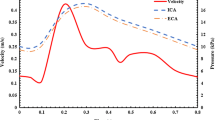
The computational simulations demonstrate that the greatest particle capture efficiency results for particle diameters within the micron range of 0.7–4 µm, specifically in regions where flow separation and vortices are at a minimum. Similar to our previous work (which did not involve the use of a magnetic stent), it was also observed that the capture efficiency of particles decreases substantially with particle diameter, especially in the superparamagnetic regime. Contrary to our previous work, using a magnetic stent tripled the capture efficiency of superparamagnetic particles. The highest capture efficiency observed for superparamagnetic particles was 78% with an 8 T magnetic field strength and 65% with a 2 T magnetic field strength when analyzing 100 nm particles. For 10 nm particles and an 8 T magnetic field strength, the particle capture efficiency was 55% and for a 2 T magnetic field strength the particle capture efficiency was observed to be 43%. Furthermore, it was found that larger magnetic field strengths, large particle diameter sizes (1 µm and above), and slower blood flow velocity improves the particle capture efficiency. The distribution of captured particles on the vessel wall along the axial and azimuthal directions is also discussed. Results for captured particles on the vessel wall along the axial flow direction showed that the particle density decreased along the axial direction, especially after the stented region. For the entrance section of the stented region, the captured particle density distribution along the axial direction is large, corresponding to the center-symmetrical distribution of the magnetic force in that section.
Conclusion
The simulation results presented in this work have shown to yield favorable capture efficiencies for micron range particles and superparamagnetic particles using magnetized implants such as the stent discussed in this work. The results presented in this work justify further investigation of MDT as a treatment technique for cardiovascular disease.














Similar content being viewed by others
Abbreviations
- BM:
-
Brownian motion
- CCA:
-
Common carotid artery
- CFD:
-
Computational fluid dynamics
- CoW:
-
Circle of Willis
- DPM:
-
Discrete phase model
- ECA:
-
External carotid artery
- FDA:
-
Food and Drug Administration
- FEM:
-
Finite element method
- FV:
-
Finite volume
- HGMS:
-
High gradient magnetic separation
- IA-MDT:
-
Implant assisted medical drug targeting
- ICA:
-
Internal carotid artery
- MDT:
-
Medical drug targeting
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Region of interest
- SA-MDT:
-
Stent assisted magnetic drug targeting
- SPION:
-
Superparamagnetic iron oxide nanoparticles
- WSS:
-
Wall shear stress
- A :
-
Experimental fit factor coefficient
- a 1 ,a 2, and a 3 :
-
Smooth particle constants
- b 1 ,b 2, and b 3 :
-
Drag coefficient constants
- B r :
-
Magnetic field strength
- C :
-
Arterial vessel capacitance
- C e :
-
Cunningham correction factor
- d :
-
Center magnet separation distance from the ICA centerline
- d c :
-
Capture distance
- d p :
-
Particle diameter
- D :
-
Diffusion coefficient
- \(\overline{\overline{D}}\) :
-
Rate of deformation tensor
- u :
-
Three-dimensional velocity vector
- u m :
-
Magnetic field induced velocity
- u p :
-
Particle parcel velocity
- ρ p :
-
Particle density
- F bi :
-
Brownian force acceleration term
- F D :
-
Drag force per unit mass
- F mx :
-
Magnetic force in the x-direction
- F my :
-
Magnetic force in the y-direction
- F x :
-
Body force acceleration term
- g x :
-
Gravitational acceleration term in the x-direction
- H :
-
Magnetic field intensity
- H x :
-
X-component magnetic field intensity
- H y :
-
Y-component magnetic field intensity
- i(t):
-
Flow rate
- k B :
-
Boltzmann constant
- L :
-
Length of the domain
- M s :
-
Saturation magnetization
- n :
-
Power law index
- N np,in :
-
Number of particles entering the domain
- N np,out :
-
Number of particles exiting the domain
- p :
-
Pressure
- Pe m :
-
Modified Peclet number
- Re :
-
Reynolds number
- R :
-
Radius of vessel
- R d :
-
Distal resistant
- R p :
-
Proximal resistance
- R mag :
-
Radius of the magnet
- R mp :
-
Radius of particle
- s :
-
Surface area of a sphere having the same volume as the particle
- S :
-
Actual area of the particle
- S 0 :
-
Spectral constant
- S n ij :
-
Spectral density
- t :
-
Time
- T :
-
Temperature
- β m :
-
Dimensionless timescale (particles to reach the wall)
- ζi :
-
Zero-mean unit-variance-independent Gaussian random number
- ρ :
-
Fluid density
- µ :
-
DYnamic viscosity
- v :
-
Kinematic viscosity
- η c :
-
Capture efficiency
- λ :
-
Time constant
- λ m :
-
Molecular mean free path
- ω :
-
Vorticity
- χ p :
-
Magnetic susceptibility
- φ :
-
Shape factor
- µ 0 :
-
Permeability of free space
- µ r :
-
Relative permeability
- µ ∞ :
-
Infinite viscosity
- τ xy = τ yx :
-
Shear Stress
- χ mp :
-
Magnetic susceptibility of the magnetic particles
- \(\dot{\gamma }\) :
-
Strain rate
References
Kulkarni, P., D. Rawtani, M. Kumar, and S. R. Lahoti. A review on the recent advancements in nanocarrier based drug delivery with a brief emphasis on the novel use of magnetoliposomes and extracellular vesicles and ongoing clinical trial research. J. Deliv. Sci. Technol. 60:102029, 2020.
Bukala, J., P. P. Buszman, J. Malachowski, L. Mazurkiewicz, and K. Sybilski. Experimental tests, FEM constitutive modeling and validation of PLGA bioresorbable polymer for stent applications. Materials. 13:2003, 2020.
Morciano, G., S. Patergnani, M. Bonora, G. Pedriali, A. Tarocco, E. Bouhamida, S. Marchi, G. Ancora, G. Anania, M. R. Wieckowski, et al. Mitophagy in cardiovascular diseases. J. Clin. Med. 9:892, 2020.
Garcimarrero-Espino, E. A., L. Figueroa-Valverde, A. Camacho-Luis, M. Rosas-Nexticapa, et al. Synthesis of new azaindeno-acetonitrile derivative with inotropic activity against heart failure model. Biointerface Res. Appl. Chem. 9:4598–4604, 2019.
Udriste, A. S., A. G. Niculescu, A. M. Grumezescu, and E. Badila. Cardiovascular stents: a review of past, current, and emerging devices. Materials. 2498(10):1–22, 2021.
Edwards, M., J. P. Kizito, and R. L. J. Hewlin. A time-dependent two species explicit finite difference computational model for analyzing diffusion in a drug eluting stented coronary artery wall: a phase I study. Proc. ASME 2022 Int. Mech. Eng. Cong. Expos. Volume 4: Biomedical and Biotechnology; Design, Systems, and Complexity. Columbus, Ohio, USA. October 30–November 3. ASME, p. V004T05A009, 2022.
Al-Jamal, T., J. Bai, J. Wang, et al. Magnetic drug targeting: preclinical in vivo studies, mathematical modeling, and extrapolation to humans. Nano Lett. 16:5652–5660, 2016.
Grief, A., and G. Richardson. Mathematical modelling of magnetically targeted drug delivery. J. Magn. Magn. Mater. 293(1):455–463, 2005.
Goya, G. F., V. Grazu, and M. R. Ibarra. Magnetic nanoparticles for cancer therapy. Curr. Nanosci. 4(1):1–16, 2008.
Hewlin, R. L. J., M. Edwards, and C. Schultz. Design and development of a traveling wave ferro-microfluidic device and system rig for potential magnetophoretic cell separation and sorting in a water-based ferrofluid. Micromachines. 14:889, 2023.
Widder, K. J., R. M. Morris, P. G. Poore, D. Howard, and A. E. Senyei. Tumor remission in yoshida sarcoma-bearing rats by selective targeting of magnetic albumin microspheres containing doxorubicin. Proc. Natl. Acad. Sci. USA. 78(1):579–581, 1981.
Ramaswamy, B., S. Kulkarni, S. Villar Pablo, et al. Movement of magnetic nanoparticles in brain tissue: mechanisms and impact on normal neuronal function. Nanomed. Nanotechnol. Biol. Med. 11(7):1821–1829, 2015.
Boghi, A., F. Russo, and F. Gori. Numerical simulation of magnetic nano drug targeting in a patient-specific coeliac trunk. J. Magn. Magn. Mater. 437:86–97, 2017.
Russo, F., A. Boghi, and F. Gori. Numerical simulation of magnetic nano drug targeting in patient-specific lower respiratory tact. J. Magn. Magn. Mater. 451:554–564, 2018.
Haverkort, J. W., K. Kenjeres, and C. R. Kleijn. Computational simulations of magnetic particle capture in arterial flows. Ann. Biomed. Eng. 37:2436–2448, 2009.
Bose, S., and M. Banerjee. Magnetic particle capture for biomagnetic fluid flow in stenosed aortic bifurcation considering particle-fluid coupling. J. Magn. Magn. Mater. 385:32–46, 2015.
Tzirtzolakis, E. E., and V. C. Lokopoulos. Biofluid flow in a channel under the action of a uniform localized magnetic field. Comp. Mech. 36(5):360–374, 2005.
Liu, Y., J. Tan, A. Thomas, H. D. Ou-Yang, and V. R. Muzykantov. The shape of things to come: importance of design in nanotechnology for drug delivery. Ther. Deliv. 3(2):181–194, 2012.
Wong, B. S., Y. G. Low, W. Xin, H. Jee-Hou, T. ChingSeong, and O. Jong Boon. 3D Finite Element Simulation of Magnetic Particle Inspection. Sustainable Utilization and Development in Engineering and Technology (STUDENT) 2010 IEEE Conference on 20–21 Nov, pp. 50-55, 2010.
Hewlin, R. L. J., and J. M. Tindall. Computational assessment of magnetic nanoparticle targeting efficiency in a simplified circle of willis arterial model. Int. J. Mol. Sci. 24:2545, 2023.
Furlani, E. P., and K. C. Ng. Analytical model of magnetic nanoparticle transport and capture in the microvasculature. Phys. Rev. E. 2006. https://doi.org/10.1103/PhysRevE.73.061919.
Iacob, G., O. Rotariu, N. J. C. Strachan, and U. O. Hafeli. Magnetizable needles and wires-modeling an efficieny way to target magnetic microspheres in vivo. Biorheology. 41:599–612, 2004.
Ning, P., C. Lanlan, K. Yang, et al. Uniform magnetic targeting of magnetic particles attracted by a new ferromagnetic biological patch. Bioelectromagnetics. 39(2):98–107, 2018.
Aviles, M. O., H. Chen, A. D. Ebner, et al. In vitro study of ferromagnetic stents for implant assisted-magnetic drug targeting. J. Magn. Mater. 311(1):306–311, 2007.
Chen, H., A. F. Ebner, A. B. Rosengart, et al. Analysis of magnetic drug carrier particle capture by a magnetizable intravascular stent: 1. Parametric study with single wire correlation. J. Magn. Magn. Mater. 284:181–194, 2004.
Diaconu, A., A. P. Chiriac, N. Tudorachi, et al. Investigation concerning the possibilities for the deposition of magnetic nanoparticles onto a metallic stent. Revue Roumaine De Chimie. 62(8–9):677–685, 2017.
Tefft, B. J., S. Uthamaraj, J. J. Harburn, O. Hlinomaz, A. Lerman, D. Dragomir-Daescu, and G. Sandhu. Magnetizable stent-grafts enable endothelial cell capture. J. Magn. Magn. Mater. 427:100–104, 2017.
Yellen, B. B., Z. G. Forbes, D. S. Halverson, et al. Targeted drug delivery to magnetic implants for therapeutic applications. J. Magn. Magn. Mater. 293(1):647–654, 2005.
Rosengart, A. J., M. D. Kaminski, H. T. Chen, P. L. Caviness, A. D. Ebner, and J. A. Ritter. Magnetizable implants and functionalized magnetic carriers: a novel approach for noninvasive yet targeted drug delivery. J. Magn. Magn. Mater. 293(1):633–638, 2005.
Ritter, J. A., A. D. Ebner, K. D. Daniel, and K. L. Stewart. Application of high gradient magnetic separation principles to magnetic drug targeting. J. Magn. Magn. Mater. 280(2–3):184–201, 2004.
Mardinoglu, A., P. J. Cregg, K. Murphy, M. Curtin, and A. Prina-Mello. Theoretical modelling of physiologically stretched vessel in magnetisable stent assisted magnetic drug targeting application. J. Magn. Magn. Mater. 323(3–4):324–329, 2011.
Forbes, Z. G., B. B. Yallen, D. S. Halverson, G. Fridman, K. A. Barbee, and G. Friedman. Validation of high gradient magnetic field based drug delivery to magnetizable implants under flow. IEEE T Bio-Med. Eng. 55(2):643–649, 2008.
Gay, M., and L. T. Zhang. Numerical studies of blood flow in healthy, stenosed, and stented carotid arteries. Int. J. Numer. Method Fluids. 61(4):453–472, 2009.
Chorny, M. F. I., B. B. Yellen, I. S. Alferiev, M. Bakay, S. Ganta, R. Adamo, M. Amiji, G. Friedman, and R. J. Levy. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc. Natl. Acad. Sci. USA. 107(18):8346–8351, 2010.
Chorny, M., I. Fishbein, I. Alferiev, and R. J. Levy. Magnetically responsive biodegradable nanoparticles enhanced adenoviral gene transfer in cultured smooth muscle and endothelial cells. Mol. Pharm. 6(5):1380–1387, 2009
Polyak, B., I. Fishbein, M. Chorny, I. Alferiev, D. Williams, B. B. Yellen, G. Freidman, and R. J. Lavy. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surface of steel stents. Proc. Natl. Acad. Sci. USA. 105(2):698–703, 2008.
Chorny, M., B. Polyak, I. S. Alferiev, K. Walsh, G. Friedman, and R. J. Levy. Magnetically driven plasmid DNA delivery with biodegradable polymeric nanoparticles. FASEB J. 21(10):2510–2519, 2007.
Chorny, M., I. Fishbein, S. Forbes, and I. Alferiev. Magnetic nanoparticles for targeted vascular delivery. Iubmb Life. 62(8):613–620, 2011.
de Vries, I. J. M., W. J. Lesterhuid, J. O. Barentsz, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 23(11):1407–1413, 2005.
Bernabeu, M. O., R. W. Nash, D. Groen, H. B. Carver, J. Hetherington, and T. Kruger. Impact of blood rheology on wall shear stress in a model of the middle cerebral artery. Interface Focus. 3:20120094, 2013.
Morales, H. G., I. Larrabide, A. J. Geers, M. L. Aguilar, and A. F. Frangi. Newtonian and non-Newtonian blood flow in coiled cerebral aneurysms. J. Biomech. 46:2158–2164, 2013.
Liu, H., L. Linfang, J. Abrigo, et al. Comparison of Newtonian and non-newtonian fluid models in blood flow simulation in patients with intracranial arterial stenosis. Front. Physiol. 12:718540, 2021.
Weddell, J. C., J. Kwack, P. I. Imoukhuede, and A. Masud. Hemodynamic analysis in an idealized artery tree: differences in wall shear stress between newtonian and non-Newtonian blood models. PLoS ONE. 10:e0124575, 2015.
Ameenuddin, M., and M. Anan. A mixture theory model for blood combined with low-density lipoprotein transport to predict early atherosclerosis regions in idealized and patient-derived abdominal aorta. J. Biomech. Eng. 2020. https://doi.org/10.1115/1.4047426.
Hewlin, R., and J. Kizito. Comparison of carotid bifurcation hemodynamics in patient-specific geometries at rest and during exercise. ASME Fluids Eng. Div. Summer Meet. 82:74, 2013.
Hewlin, R. Transient Cardiovascular Hemodynamics in a Patient-Specific Arterial System. New York: ProQuest Dissertation Publishing, 2015.
Stanley, N., A. Ciero, W. Timms, and R.L.J. Hewlin. Development of 3-D printed optically clear rigid anatomical vessels for particle image velocimetry analysis in cardiovascular flow. Proc. ASME 2019 Int. Mech. Eng. Congr. Expos.. Volume 7: Fluids Engineering. Salt Lake City, Utah, USA. November 11–14. V007T08A004. ASME, 2019.
Stanley, N., A. Ciero, W. Timms, and R. L. J. Hewlin. A 3-D printed optically clear rigid diseased carotid bifurcation arterial mock vessel model for particle image velocimetry analysis in pulsatile flow. ASME Open J. Eng. ASME. 2023. https://doi.org/10.1115/1.4056639.
Hewlin, J. R. L., and J. P. Kizito. Development of an experimental and digital cardiovascular arterial model for transient hemodynamic and postural change studies: a preliminary framework analysis. Cariodvasc. Eng. Tech. 9:1–31, 2018.
Hewlin, J. R. L., and J. P. Kizito. Evaluation of the effect of simplified and patient-specific arterial geometry on hemodynamic flow in stenosed carotid bifurcation arteries. ASME Early Career Techn. J. 10:39–44, 2011.
Gharahi, H., B. Zambrano, D. Zhu, J. Dermarco, and B. Seungik. Computational fluid dynamic simulation of human carotid artery bifurcation based on anatomy and volumetric blood flow rate measured with magnetic resonance imaging. Int. J. Adv. Eng. Sci. 8(1):40–60, 2016.
Furlani, E. J., and E. P. Furlani. A model for predicting magnetic targeting of multifunctional particles in the microvasculature. J. Magn. Magn. Mater. 312:187–193, 2007.
Hewlin, R. L. J., M. Edwards, and M. Smith. A 2D transient computational multi-physics model for analyzing magnetic and non-magnetic particle (red blood cells and E. Coli bacteria) dynamics in a travelling wave ferro-magnetic microfluidic device for potential cell separation and sorting. ASME J. 2023. https://doi.org/10.1115/1.4062571.
Wang, S., Y. Zhou, J. Tan, et al. Computational modelling of magnetic nanoparticle targeting to stent surface under high gradient field. Comput. Mech. 53(3):403–412, 2014.
Takayasu, M., R. Gerber, and F. J. Friedlaender. Magnetic separation of submicron particles. IEEE Trans. Magn. 19(5):2112–2114, 1983.
Hewlin, J. R. L., A. Ciero, and J. P. Kizito. Development of a two-way coupled Eulerian-Lagrangian computational magnetic nanoparticle targeting model for pulsatile flow in a patient-specific diseased left carotid bifurcation artery. Cardiovasc. Eng. Technol. 10(2):299–313, 2019.
Lunnoo, T., and T. Puangmali. Capture efficiency of biocompatible magnetic nanoparticles in arterial flow: a computer simulation for magnetic drug targeting. Nanoscale Res. Lett. 10(426):1–11, 2015.
Morsi, S. A., and A. J. Alexander. An investigation of particle trajectories in two-phase flow systems. J. Fluid Mech. 55(2):193–208, 1972.
Haider, A., and O. Levenspiel. Drag coefficient and terminal velocity of spherical and nonspherical particles. Powder Technol. 58:63–70, 1989.
Ounis, H., G. Ahmadi, and J. B. McLaughlin. Brownian diffusion of submicrometer particles in viscous sublayer. J. Colloid Interface Sci. 143(1):266–277, 1991.
Bose, S., A. Datta, R. Ganguly, and M. Banerjee. Lagrangian magnetic particle tracking through stenosed artery under pulsatile flow condition. J. Nano Eng. Med. 4(3):1–10, 2014.
Zaremba, L. A. Guidance for Industry and FDA Staff: Criteria for Significant Risk Investigations of Magnetic Resonance Diagnostic Devices. Washington DC: Center for Devices and Radiological Health, 2003.
Wang, J., J. D. Byrne, M. E. Napier, and J. M. DeSimone. More effective nanomedicine through particle design. Small. 7(14):1919–1931, 2011.
Petros, R. A., and J. M. DeSimone. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 9(8):615–627, 2010.
Cheung, S. C., K. K. Wong, G. H. Yeoh, et al. Experimental and numerical study on the hemodynamics of stenosed carotid bifurcation. Australas. Phys. Eng. Sci. Med. 33(4):319–328, 2011.
Vetel, J., A. Garon, and S. Pelletier. Lagrangian coherent structures in the human carotid artery bifurcation. Exp. Fluids. 46(6):1067–1079, 2009.
Buchmann, A. C., M. C. Jeremy, and J. Soria. Tomographic particle image velocimetry investigation of the flow in a modeled human carotid artery bifurcation. Exp. Fluids. 50(4):1131–1151, 2011.
Sui, B., P. Gao, Y. Lin, B. Gao, L. Liu, and J. An. Assessment of wall shear stress in the common carotid artery of healthy subjects using 3.0-tesla magnetic resonance. Acta Radiol. 49(4):442–449, 2008.
Xiao, L., S. Beibei, Z. Huilin, et al. Retrospective study of hemodynamic changes before and after carotid stenosis formation by vessel surface repairing. Nature. 5493:1–8, 2018.
Shubayev, V. I., T. R. Pisanic, and S. Jin. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 61:467–477, 2009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this work declare no conflict of interests.
Research involving human and animal rights
No animal studies were carried out by the authors for this article. No human studies were carried out by the authors for this article.
Additional information
Associate Editor Sarah Vigmostad oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hewlin, R.L., Smith, M. & Kizito, J.P. Computational Assessment of Unsteady Flow Effects on Magnetic Nanoparticle Targeting Efficiency in a Magnetic Stented Carotid Bifurcation Artery. Cardiovasc Eng Tech 14, 694–712 (2023). https://doi.org/10.1007/s13239-023-00681-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-023-00681-3