Abstract
Purpose
Preeclampsia (PE) is a pregnancy complication of abnormally elevated blood pressure and organ damage where endothelial function is impaired. Wall shear stress (WSS) strongly effects endothelial cell morphology and function but in PE the WSS values are unknown. WSS calculations from ultrasound inaccurately assume cylindrical arteries and patient specific computational fluid dynamics (CFD) typically require time-consuming 3D imaging such as CT or MRI.
Methods
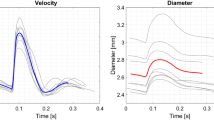
Two-dimensional (2D) B-mode ultrasound images were lofted together to create simplified three-dimensional (3D) geometries of the brachial artery (BA) that incorporate artery curvature and non-circular cross sections. This process was efficient and on average took 120 ± 10 s. Patient specific CFD was then performed to quantify BA WSS for a small cohort of PE (n = 5) and normotensive pregnant patients (n = 5) and compared against WSS calculations assuming a cylindrical artery.
Results
For several WSS metrics (time averaged WSS (TAWSS), peak systolic WSS, oscillatory shear index (OSI), OSI/TAWSS and relative residence time) CFD on the simplified arterial geometries calculated large spatial differences in WSS that assuming a cylindrical artery cannot calculate. Bland–Altman and intra-class correlation (ICC) analyses found assuming a cylindrical artery both underestimated (p < 0.05) and had poor agreement (ICC < 0.5) with the maximum WSS values from CFD. WSS values that were abnormal compared to the normotensive patients (OSI = 0.014 ± 0.026) appear related to the pregnancy complications fetal growth restriction (n = 2, OSI = 0.14, 0.25) and gestational diabetes (n = 1, OSI = 0.23).
Conclusion
Creating 3D artery geometries from 2D ultrasound images can be used for CFD simulations to calculate WSS from ultrasound without assuming cylindrical arteries. This approach requires minimal time for both medical imaging and CFD analysis.





Similar content being viewed by others
Abbreviations
- CFD:
-
Computational fluid dynamics
- PE:
-
Preeclampsia
- WSS:
-
Wall shear stress
- BA:
-
Brachial artery
- NT:
-
Normotensive
- TAWSS:
-
Time average wall shear stress
- OSI:
-
Oscillatory shear index
- RRT:
-
Relative residence time
References
Arzani, A., G. Y. Suh, R. L. Dalman, and S. C. Shadden. A longitudinal comparison of hemodynamics and intraluminal thrombus deposition in abdominal aortic aneurysms. Am. J. Physiol. Hear. Circ. Physiol. 307:H1786–H1795, 2014.
Ballermann, B. J., A. Dardik, E. Eng, and A. Liu. Shear stress and the endothelium. Kidney Int 54:S100–S108, 1998.
Bluestein, D., L. Niu, R. T. Schoephoerster, and M. K. Dewanjee. Steady flow in an aneurysm model: correlation between fluid dynamics and blood platelet deposition. 1996. https://asmedigitalcollection.asme.org/biomechanical/article-pdf/118/3/280/5766734/280_1.pdf.
Boekhoven, R. W., R. G. P. Lopata, M. R. van Sambeek, F. N. van de Vosse, and M. C. M. Rutten. A novel experimental approach for three-dimensional geometry assessment of calcified human stenotic arteries in vitro. Ultrasound Med. Biol. 39:1875–1886, 2013.
Burton, G. J., C. W. Redman, J. M. Roberts, and A. Moffett. Pre-eclampsia: pathophysiology and clinical implications. BMJ 366:l2381, 2019.
Coolbaugh, C. L., E. C. Bush, C. F. Caskey, B. M. Damon, and T. F. Towse. FloWave.US: validated, open-source, and flexible software for ultrasound blood flow analysis. J. Appl. Physiol. 121:849–857, 2016.
Guerci, B., P. Böhme, A. Kearney-Schwartz, F. Zannad, and P. Drouin. Endothelial dysfunction and type 2 diabetes. Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab 27:436–447, 2001.
Guerci, B., A. Kearney-Schwartz, P. Böhme, F. Zannad, and P. Drouin. Endothelial dysfunction and type 2 diabetes: part 1: physiology and methods for exploring the endothelial function. Diabetes Metab 27:425–434, 2001.
Hausberg, M., K. Kisters, M. Kosch, K. H. Rahn, and M. Barenbrock. Flow-mediated vasodilation and distensibility of the brachial artery in renal allograft recipients. Kidney Int. 55:1104–1110, 1999.
Himburg, H. A., D. M. Grzybowski, A. L. Hazel, J. A. Lamack, X.-M. Li, M. H. Friedman, and M. H. Friedman. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability Downloaded from. Am. J. Physiol. Hear. Circ. Physiol. 286:1916–1922, 2004.
Hogan, M. C., K. J. Foreman, M. Naghavi, S. Y. Ahn, M. Wang, S. M. Makela, A. D. Lopez, R. Lozano, and C. J. Murray. Maternal mortality for 181 countries, 1980-2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 375:1609–1623, 2010.
Humphrey, J. D., P. Di Achille, G. Tellides, and C. A. Figueroa. A haemodynamic predictor of intraluminal thrombus formation in abdominal aortic aneurysms. Proc. R. Soc. A Math. Phys. Eng. Sci. 2014. https://doi.org/10.1098/rspa.2014.0163.
Joly, F., G. Soulez, S. Lessard, C. Kauffmann, and I. Vignon-Clementel. A cohort longitudinal study identifies morphology and hemodynamics predictors of abdominal aortic aneurysm growth. Ann. Biomed. Eng. 48:606–623, 2020.
Katritsis, D., L. Kaiktsis, A. Chaniotis, J. Pantos, E. P. Efstathopoulos, and V. Marmarelis. Wall shear stress: theoretical considerations and methods of measurement. Prog. Cardiovasc. Dis. 49:307–329, 2007.
Kheyfets, V. O., L. Rios, T. Smith, T. Schroeder, J. Mueller, S. Murali, D. Lasorda, A. Zikos, J. Spotti, J. J. Reilly, and E. A. Finol. Patient-specific computational modeling of blood flow in the pulmonary arterial circulation. Comput. Methods Progr. Biomed. 120:88–101, 2015.
Kuklina, E. V., C. Ayala, and W. M. Callaghan. Hypertensive disorders and severe obstetric morbidity in the United States LEVEL OF EVIDENCE: III. Obstet Gynecol 113(6):1299–1306, 2009.
Lu, D., and G. S. Kassab. Role of shear stress and stretch in vascular mechanobiology. J. R. Soc. Interface 8:1379–1385, 2011.
Magness, R. R., C. E. Shaw, T. M. Phernetton, J. Zheng, and I. M. Bird. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am. J. Physiol. Heart Circ. Physiol. 272(4):H1730–H1740, 1997.
Magness, R. R., J. A. Sullivan, T. M. Phernetton, I. M. Bird, Y. Li, and I. M. Bird. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NOx. Am. J. Physiol. Heart Circ. Physiol. 280(4):H1692–H1698, 2001.
Mcveigh, G. E., G. M. Brennan, G. D. Johnston, B. J. Mcdermott, L. T. Mcgrath, W. R. Henry, J. W. Andrews, and J. R. Hayes. Impaired endothelium-dependent and independent vasodilation in patients with Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35:771–776, 1992.
Mynard, J. P., B. A. Wasserman, and D. A. Steinman. Errors in the estimation of wall shear stress by maximum Doppler velocity. Atherosclerosis 227:259–266, 2013.
Placental bed disorders: basic science and its translation to obstetrics—Google Booksat. https://books.google.com/books?hl=en&lr=&id=uMuQ4Q1nR4MC&oi=fnd&pg=PA229&dq=Tjoa+M,+et+al.+Front+Biosci+2007%3B12:2395-2402.&ots=-C5JOUdcoC&sig=2CzwmfFSC05H6wI9eu9dutPFvOo#v=onepage&q&f=false.
Prakash, S., and C. R. Ethier. Requirements for mesh resolution in 3D computational hemodynamics. J. Biomech. Eng. 123:134–144, 2001. https://doi.org/10.1115/1.1351807.
Roberts, J. Endothelial dysfunction in preeclampsia. Semin. Reprod. Med. 16:5–15, 1998.
Silber, H. A., P. Ouyang, D. A. Bluemke, S. N. Gupta, T. K. Foo, and J. A. C. Lima. Why is flow-mediated dilation dependent on arterial size? Assessment of the shear stimulus using phase-contrast magnetic resonance imaging. Am. J. Physiol. Circ. Physiol. 288:H822–H828, 2005.
Taylor, C. A., and D. A. Steinman. Image-based modeling of blood flow and vessel wall dynamics: applications, methods and future directions: sixth international bio-fluid mechanics symposium and workshop, March 28–30, 2008 Pasadena, California. Ann. Biomed. Eng. 38:1188–1203, 2010.
Tomimatsu, T., K. Mimura, S. Matsuzaki, M. Endo, K. Kumasawa, and T. Kimura. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int. J. Mol. Sci. 20:4246, 2019.
Updegrove, A., N. M. Wilson, J. Merkow, H. Lan, A. L. Marsden, and S. C. Shadden. SimVascular: an open source pipeline for cardiovascular simulation. Ann. Biomed. Eng. 45:525–541, 2017.
Whiting, C. H., and K. E. Jansen. A stabilized finite element method for the incompressible Navier-Stokes equations using a hierarchical basis. Int. J. Numer. Methods Fluids 35:93–116, 2001.
Wiputra, H., C. K. Chen, E. Talbi, G. L. Lim, S. M. Soomar, A. Biswas, C. N. Z. Mattar, D. Bark, H. L. Leo, and C. H. Yap. Human fetal hearts with tetralogy of fallot have altered fluid dynamics and forces. Am. J. Physiol. Hear. Circ. Physiol. 315:H1649–H1659, 2018.
Wiputra, H., G. L. Lim, K. C. Chua, R. Nivetha, S. M. Soomar, A. Biwas, C. N. Z. Mattar, H. L. Leo, and C. H. Yap. Peristaltic-like motion of the human fetal right ventricle and its effects on fluid dynamics and energy dynamics. Ann. Biomed. Eng. 45:2335–2347, 2017.
Zhou, X., L. Yin, L. Xu, and F. Liang. Non-periodicity of blood flow and its influence on wall shear stress in the carotid artery bifurcation: an in vivo measurement-based computational study. J. Biomech. 2020. https://doi.org/10.1016/j.jbiomech.2020.109617.
Acknowledgments
This investigation was supported by R&D funding from the University of Wisconsin – Madison Obstetrics and Gynecology department and by the NIH Ruth L. Kirschstein National Research Service Award T32 HL 007936 from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center (RP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor David Elad oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pewowaruk, R.J., Racine, J., Iruretagoyena, J.I. et al. Ultrasound Based Computational Fluid Dynamics Assessment of Brachial Artery Wall Shear Stress in Preeclamptic Pregnancy. Cardiovasc Eng Tech 11, 760–768 (2020). https://doi.org/10.1007/s13239-020-00488-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-020-00488-6