ABSTRACT
Although the molecular basis of flowering time control is well dissected in the long day (LD) plant Arabidopsis, it is still largely unknown in the short day (SD) plant rice. Rice flowering time (heading date) is an important agronomic trait for season adaption and grain yield, which is affected by both genetic and environmental factors. During the last decade, as the nature of florigen was identified, notable progress has been made on exploration how florigen gene expression is genetically controlled. In Arabidopsis expression of certain key flowering integrators such as FLOWERING LOCUS C (FLC) and FLOWERING LOCUS T (FT) are also epigenetically regulated by various chromatin modifications, however, very little is known in rice on this aspect until very recently. This review summarized the advances of both genetic networks and chromatin modifications in rice flowering time control, attempting to give a complete view of the genetic and epigenetic architecture in complex network of rice flowering pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rice flowering time (heading date), which is affected by both endogenous and exogenous factors, is an important agronomic trait for regional and seasonal adaption. Heading on a proper time is the most critical step for grain production. Precocious flowering reduces the vegetative phase and leads to reduction of biological yield. On the other hand, delayed flowering could cause low seed setting percentage in cold late autumn or delay next planting season, which both results in production loss.
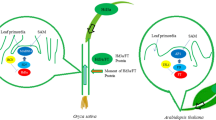
Florigen is produced in the leaf under inductive day length conditions and transported to the shoot apex where it triggers flowering transition (Cajlachjan, 1937; Corbesier et al., 2007; Tamaki et al., 2007). Unlike only one florigen gene FLOWERING LOCUS T (FT) in Arabidopsis, rice evolves two florigen genes, Heading date 3a (Hd3a) and RICE FLOWERING LOCUS T 1 (RFT1), and at least two flowering pathways are developed to control the expression of florigens, the Heading date 1 (Hd1) pathway which is conserved between rice and Arabidopsis, and the Early heading date 1 (Ehd1) pathway which is unique to rice (Doi et al., 2004) (Fig. 1). Numerous studies reveal that a large number of rice genes regulate flowering time through the two flowering integrators.
Comparison of core-flowering-pathways in rice and Arabidopsis. OsGI/GI-Hd1/CO-Hd3a/FT pathway is conserved between rice and Arabidopsis. CO. accelerates flowering under LD, however, Hd1 promotes flowering under SD and represses it under LD. Besides, Ghd7 and Ehd1 in rice, and FLC in Arabidopsis are unique flowering integrators, respectively. FLC and Ghd7 are major flowering suppressors, while Ehd1 acts as a flowering promoter
In Arabidopsis, some flowering regulators such as FLC and FT are reported to be regulated by various chromatin modifications (He, 2009; Liu et al., 2010). However, little is known in rice in this field. Recently, we characterized a major histone methyltransferase (HMTase) gene SET DOMAIN GENE 724 (SDG724), which is required for Histone H3 lysine 36 (H3K36) methylation, promotes rice heading, indicating that rice flowering could also be regulated by chromatin modifications (Sun et al., 2012). In the past two years, more and more molecular genetic studies gave the clues on the chromatin modification mechanism in rice flowering pathways, we summarize here most recent advances towards understanding of genetic networks and epigenetic chromatin modifications in rice flowering time control.
TWO FLORIGEN GENES Hd3a AND RFT1 IN RICE
Florigen, which has been hypothesized by many physiological studies, is believed to be produced in leaves by the inductive photoperiod, then moves to the shoot apical meristem (SAM) and triggers flowering transition. But this florigen has been eluded identification since it was first proposed for 70 years (Cajlachjan, 1937). In 2007, it was firstly revealed that FT encoded protein in Arabidopsis, is a leaf-derived long-distance signal directed to floral transition (Corbesier et al., 2007).
In rice, there are 13 FT homologs in the genome (Chardon and Damerval, 2005), Hd3a and RFT1 are two of them which were confirmed to act as florigen genes (Komiya et al., 2008; Komiya et al., 2009; Tamaki et al., 2007). By fusing Hd3a or RFT1 with GFP, it was demonstrated that Hd3a or RFT1 protein was expressed in vascular tissue of leaves, and could be moved to SAM where they started flowering induction. As Hd3a-GFP was only detected in the SAM of plants grown under short day conditions (SD), RFT1-GFP was merely detected under long day conditions (LD) (Komiya et al., 2009; Tamaki et al., 2007). On the other hand, Hd3a-RNAi (RNA interference) plants significantly delayed heading date under SD but not LD, RFT1-RNAi plants flowering was obviously delayed under LD but not SD oppositely. Furthermore, rice with knockout of both Hd3a and RFT1 caused at least 300 days late flowering under both SD and LD (Komiya et al., 2009). All these data demonstrated that, unlike Arabidopsis, rice has two florigen genes, Hd3a and RFT1, Hd3a is responsible for flowering under inductive SD, whereas RFT1 is responsible for flowering transition under non-inductive LD. Although Hd3a and RFT1 are located in some chromosome and separated by only 11.5 kb in the genome, the fine-tuning of long day flowering by the H3K36me2/3 level of RFT1 but not Hd3a via SDG724, therefore, RFT1 and Hd3a which have functionally diverged to control flowering time under LD and SD conditions are partly due to a fine-tuned epigenetic mechanism (Sun et al., 2012).
FLORIGEN REGULATED NETWORK
How flowering pathways are regulated differs in plants. In Arabidopsis, flowering is controlled by a small number of large-effect genes such as FLC (Salome et al., 2011), whereas in maize is controlled by many additive small-effect quantitative trait loci (QTLs) (Buckler et al., 2009). Interestingly, rice combines both regulatory manners, including a few large-effect factors, such as Hd1, Ehd1, and Grain number, plant height andheading date 7 (Ghd7), in addition to some small-effect QTLs and genes (Ebana et al., 2011; Tsuji et al., 2013) (Table 1).
So far, quite a number of QTLs controlling rice heading date (Hd) were identified and characterized using different segregating populations derived from crossing a japonica cultivar (Nipponbare) and an indica cultivar (Kasalath) (Lin et al., 1998; Yano et al., 1997). These QTLs include the major loci controlling photoperiodic flowering responses, Hd1 (Yano et al., 1997; Yano et al., 2000), Hd2/Ghd7.1/OsPRR37 (Oryza sativa Pseudo-Response Regulator 37) (Koo et al., 2013; Liu et al., 2013; Shibaya et al., 2011; Yamamoto et al., 2000; Yan et al., 2013), Hd3a (Kojima et al., 2002), Hd4/Ghd7 (Ghd7 for short) (Koo et al., 2013; Xue et al., 2008), Hd5/Days to heading 8/Grain number, Plant height, and Heading date 8/Late Heading Date 1 (Hd5/DTH8/Ghd8/LHD1) (Dai et al., 2012; Fujino et al., 2013; Yan et al., 2011). Furthermore, backcross progenies derived from the same original cross allowed identification of other QTLs, such as Hd6/CK2 (CASEIN KINASE 2) (Ogiso et al., 2010; Takahashi et al., 2001; Yamamoto et al., 2000), Hd14/Ehd1 (Doi et al., 2004), Hd16/EL1 (Early flowering 1) (Dai and Xue, 2010; Hori et al., 2013; Shibaya et al., 2011), Hd17/OsELF3/EF7/OsEF3/Hd3b (Hd17/Oryza sativa Early Flowering 3/Early Flowering 7/Oryza sativa Early Flowering 3/Hd3b, OsELF3 for short) (Hori et al., 2012; Matsubara et al., 2012; Saito et al., 2012; Yang et al., 2013; Zhao et al., 2012). Additionally, using rice near isogenic lines and mutants, more genes implicated in controlling flowering time have been identified and positioned into a regulatory network (Brambilla and Fornara, 2013; Itoh and Izawa, 2013; Tsuji et al., 2011, 2013) (Fig. 2).
Complex flowering time control network in rice. Rice flowering network is formed by two florigen genes Hd3a and RFT1, and four regulation modules, including Hd1-dependent pathway, Ehd1-dependent pathway, crosstalk between Hd1 and Ehd1 pathway, and flowering regulators independent of Hd1 and Ehd1. The first three signals come together to regulate Hd1 and Ehd1 and affect florigen gene expression; the last module may directly control the expression of florigen genes independent of Hd1 and Ehd1. Besides, expressions of Ehd3, RID1, OsMADS50, Hd3a, and RFT1 can be affected by H3K36me2/3; OsLFL1 and OsLF transcriptions can be mediated by H3K27me3. Finally, all those florigen signals are transported from leaves to SAM and trigger flowering transition there. All the gene names for short are showed in Table 1
Hd1-DEPENDENT PATHWAY
There is a similar molecular system for florigen control in Arabidopsis and rice (Izawa, 2007; Tsuji et al., 2011). Hd1 and Hd3a in rice are homologs of CONSTANS (CO) and FT in Arabidopsis, respectively. As in Arabidopsis, Hd1 acts upstream of Hd3a (Kojima et al., 2002; Yano et al., 2000), and overexpression of a rice ortholog of Arabidopsis GIGANTEA (GI) which acts upstream of CO, namely OsGI, increased the expression of Hd1 in the transgenic plants, followed by suppressing Hd3a expression, resulting in late flowering under both SD and LD (Hayama et al., 2003). Differently, CO merely promotes FT expression, Hd1 plays a more enigmatic role in rice, which promotes flowering under SD, but represses flowering under LD (Hayama et al., 2003; Komiya et al., 2008; Lin et al., 2000; Tamaki et al., 2007). These results indicate that the core photoperiodic pathway composed of the three key flowering genes OsGI/GI-Hd1/CO-Hd3a/FT is conserved between rice and Arabidopsis, but its function has diverged during evolution to produce opposite flowering responses. While the photoperiodic pathway in Arabidopsis merely accelerates flowering under LD, in rice, it promotes flowering under SD and represses flowering under LD (Takahashi and Shimamoto, 2011).
The reversible mechanism that Hd1 functions as either an activator or suppressor of Hd3a involves the action of the red-light photoreceptor phytochrome B (phyB), since mutations in phyB or phytochrome chromophore synthesis, such as photoperiod sensitivity 5 (se5), attenuate this conversion and maintain Hd1 as an activator under any photoperiodic conditions. On the other hand, though Hd1-overexpressing plants delay flowering, Hd1 protein levels in these plants are not significantly altered (Andres et al., 2009; Ishikawa et al., 2011; Izawa et al., 2002), thus it is speculated that LD light signals may modify the protein of Hd1 or Hd1 complex through phytochrome but not its expression levels, and convert it into a repressor of flowering. Therefore, uncovering of the biochemical function of Hd1 protein and the molecular nature of its dual activity will provide exciting insight into the control of photoperiodic flowering in rice.
Recently, it is deduced that Hd1 protein activity is possible affected by an additional posttranslational regulatory factor, Hd6, which encodes a CK2 α-subunit (Ogiso et al., 2010; Takahashi et al., 2001). The delay flowering effect of Hd6 is observed only when Hd1 is functional, however, Hd1 is not phosphorylated by Hd6 in vitro (Ogiso et al., 2010), suggesting that Hd6 phosphorylates unknown substrates that cooperate with Hd1 in the LD floral suppression pathway.
Ehd1 DEPENDENT PATHWAY
In 2004, a novel regulatory Ehd1-pathway which is not presented in Arabidopsis, is discovered in rice (Doi et al., 2004). Ehd1, encoding a B-type response regulator, is a floral promoter, and rice variety Taichung 65 (T65) without functional Ehd1 allele delays flowering under both LD and SD (Doi et al., 2004). As it has been shown that Ehd1 contributes to flowering time by its expression levels (Takahashi et al., 2009), thus fine-tuning of Ehd1 expression is crucial for rice flowering at suitable time, and several flowering regulators have been identified to participate in this regulation.
Ghd7, which is important for increasing rice productivity and adaptability, is a major regulator of Ehd1 and could delay flowering by repressing Ehd1 under LD (Takahashi et al., 2009; Xue et al., 2008). As Ghd7 encodes a CCT (CONSTANS, CO-like, and TOC1) domain protein, which shows very low homology to Arabidopsis genome, the Ghd7-Ehd1 may be a unique pathway in rice (Koo et al., 2013; Xue et al., 2008). Further study shows that Ghd7 and Ehd1 can respectively set a daylength threshold for Hd3a expression, which is usually observed in SD plants but not in LD plants (Itoh et al., 2010; Takimoto and Ikeda, 1961), and this capacity of discernment in critical day length in rice greatly enriches the daylength-dependent regulated mechanism of florigen gene expression.
Until now, at least three genes, Early heading date 3 (Ehd3), ELF3, and Hd16/EL1, were identified to control Ghd7 expression in Ehd1-pathway. Ehd3 encodes a plant homeodomain (PHD) finger protein and is identified as one repressor of Ghd7. Generally, Ghd7 transcript reaches its highest level after seeding for two weeks, and then the expression is gradually reduced to a basal level, but in ehd3 mutants, Ghd7 expression level is always higher and delays heading date for more than one year under LD. Interestingly, under SD, Ehd3 could promote Ehd1 expression regardless of Ghd7, suggesting a perplexed role of Ehd3 (Matsubara et al., 2011).
ELF3 in Arabidopsis is responsible for generating circadian rhythm and regulating photoperiodic flowering, consistently, its homolog in rice OsELF3 is also required to sustain the robust oscillation, and lesions in OsELF3 delay flowering under both SD and LD (Saito et al., 2012; Yang et al., 2013; Zhao et al., 2012). Under SD, OsELF3 promotes flowering mainly by repressing Ghd7, because late flowering of oself3 mutants can be rescued if Ghd7 but not Hd1 is mutated. Under LD, oself3 mutants increase OsGI and Ghd7 expression, thus up-regulate Hd1 and repress Ehd1 expression, respectively, indicating that OsELF3 influences photoperiodic flowering in both Hd1 and Ehd1 pathways (Brambilla and Fornara, 2013; Saito et al., 2012).
Hd16/EL1, encoding a casein kinase I protein, is associated with the gibberellin-mediated flowering transition (Dai and Xue, 2010). Deficient in Hd16 weakens rice photoperiod sensitivity, but increases Ehd1, Hd3a, and RFT1 expression under LD. Though the expression level of Ghd7 is not significantly altered in el1 mutants, the biochemical data indicate that Hd16 acts as a flowering repressor by phosphorylation of Ghd7 (Dai and Xue, 2010; Kwon et al., 2013).
OsLFL1 (Oryza sativa LEC2 and FUSCA3 Like 1) encodes a putative B3 transcription factor, knockdown of OsLFL1 does not affect flowering time, while ectopic overexpression of OsLFL1 decreases Ehd1 expression and results in late flowering (Peng et al. 2007, 2008). OsLFL1 is controlled by two members of MIKC-type MADS-box family, OsMADS50 and OsMADS56. Both osmads50 mutants and OsMADS56-overexpressing plants, which produce increased OsLFL1 expression, show late flowering phenotype (Lee et al., 2004; Ryu et al., 2009). Interestingly, OsMADS56 can interact with OsMADS50 in vitro, suggesting that the two MADS-box members tend to form a heterodimer complex and function antagonistically through OsLFL1-Ehd1 pathway under LD (Ryu et al., 2009).
As mentioned in Hd1-pathway, phytochrome is probably a primary cause of Hd1-dependent suppression of rice flowering, but underlying molecular mechanism of phytochrome in Ehd1-pathway is not well understood. Recent studies showed that SE5 and phyB also suppress Ehd1 expression, and the phyB-mediated suppression of Ehd1 is confirmed to be repressed by a CONSTANS-like (COL) gene OsCOL4 (Oryza sativaCONSTANS-like4) (Andres et al., 2009; Komiya et al., 2009; Lee et al., 2010). OsCOL4 expression is decreased in osphyB mutants, and osphyB oscol4 double mutants flower is similar to osphyB single mutants, indicating that OsCOL4 functions downstream of OsphyB (Lee et al., 2010).
Besides the above regulators, Ehd1 expression is also modulated by other four flowering factors independently. Indeterminate 1 (ID1) is one of them, which expresses in leaf but induces flowering in the shoot meristem. ID1 has been once thought to be involved in the florigen synthesis in maize (Colasanti et al., 2006; Colasanti et al., 1998), and its regulated mechanism has been exhibited in rice. Lesions in rice RID1 (Early heading date 2/OsINDETERMINATE 1/Rice INDETERMINATE 1, Ehd2/OsID1/RID1, RID1 for short) lead to extremely late flowering phenotype, as well as decreased expression of Ehd1 and downstream florigen genes under both SD and LD (Matsubara et al., 2008; Park et al., 2008; Wu et al., 2008).
Ehd4 (Early heading date 4), encoding a CCCH-type zinc finger transcriptional regulator, is expressed mostly in immature leaves and shows a similar diurnal expression pattern of Ehd1 under both SD and LD. Ehd4 up-regulates the expression of the florigen genes Hd3a and RFT1 through Ehd1. Strikingly, Ehd4 is highly conserved in both wild rice and cultivated rice, but homologs cannot be found in other species, suggesting that Ehd4 is unique flowering regulator in Oryza genus differed from other grass members during evolution (Gao et al., 2013).
OsMADS51 is another MADS box gene, other than OsMADS50 and OsMADS56, it acts downstream of OsGI, transmits a promotion signal from OsGI to Ehd1 under SD. Though its null mutants showed late flowering phenotype followed by decreased expression of Ehd1 and Hd3a, ectopic expression of OsMADS51 causes early flowering, accompanying with increased expression Ehd1 and Hd3a (Kim et al., 2007).
Hd5/DTH8/Ghd8/LHD1 encodes a putative HEMEACTIVATOR PROTEIN 3 (HAP3) subunit of a CCAAT-box binding protein (HAP complex) that binds to CCAAT boxes in yeast and animals. Similar to Hd1, Hd5/DTH8/Ghd8/LHD1 delayed flowering in rice under LD and promotes flowering under SD, but by regulating expression of Ehd1 (Dai et al., 2012; Lin et al., 2003; Wei et al., 2010; Yan et al., 2011).
Most interestingly, though Hd5/DTH8/Ghd8/LHD1 suppresses rice heading though Ehd1, genetic analysis implies that Hd5 requires functional Hd1 to repress flowering under LD (Nonoue et al., 2008), rising a question what is the relationship between Hd1 and Ehd1. Recent findings indicate that transcript level of Ehd1 is down-regulated in Hd1-overexpression transgenic lines, suggesting that, to some degree, Hd1 is an upstream regulator of Ehd1 expression, but how this crosstalk works is still undefined (Ishikawa et al., 2011).
FLOWERING REGULATORS INDEPENDENT OF Hd1 AND Ehd1
Besides Ehd1, T65 also bears a loss-of-function allele of Hd1, but it could still flower in time and serves as a commercial rice variety, so there are must some other regulators independent of Hd1 and Ehd1 in rice flowering network (Doi et al., 2004). OsCO3 and DTH2 are two of them, and promote flowering by regulating florigen genes. Though both of them are COL genes, they function under different photoperiodic conditions. Expressions of Hd3a and FT-like genes are decreased in the OsCO3-overexpressing plants under SD without altered expression of other florigen upstream regulators, suggesting that OsCO3 primarily controls flowering time under SD by negatively regulating the expression of florigen genes, independent of other known SD-promotion pathways (Kim et al., 2008). For DTH2, both association analysis and transgenic experiments indicate that two functional nucleotide polymorphisms that correlated with early heading and increased reproductive fitness under natural LD in northern Asia. Further combined population genetics and network analyses suggest that DTH2 probably represents a target of artificial selection for adaptation to LD during rice domestication and improvement, demonstrating an important role of minor effect quantitative trait loci in crop adaptation and breeding (Wu et al., 2013).
Although some PRR genes are major components of the circadian oscillator, a rice PRR gene Hd2/Ghd7.1/OsPRR37 may down-regulate Hd3a expression independent of any known pathways to suppress flowering under LD. As lesions in Hd2/Ghd7.1/OsPRR37 cause early flowering phenotype, the japonica varieties harboring nonfunctional alleles of both Ghd7 and Hd2/Ghd7.1/OsPRR37 flower extremely early under natural LD, and make these varieties adapt to the northernmost rice cultivation regions. Further study implied that natural variations in Hd2/Ghd7.1/OsPRR37 have contributed to the expansion of rice cultivation to temperate and cooler regions (Koo et al., 2013; Liu et al., 2013; Yan et al., 2013).
Different from Hd2/Ghd7.1/OsPRR37, OsDof12 is LD-specific flowering repressor and encodes a DNA-binding with one finger (Dof) transcription factor which is involved in a variety of biological processes of plants. The transcriptions of OsDof12 can express at different development stages, but strongly inhibited by dark treatment. OsDof12-overexpressing plants flower earlier in consistent with the up-regulation of Hd3a independent of other flowering genes under LD but not SD (Li et al., 2009).
CHROMATIN MODIFICATIONS REGULATE FLOWERING IN RICE
Chromatin, which is composed by complexing DNA with histone, carries not only genetic, but also epigenetic information. In Arabidopsis, the expression of a major flowering repressor FLC is regulated by a number of active and repressive chromatin modifications, such as histone tails methylation, acetylation, ubiquitination etc. In addition, histone modifications can also directly regulate the expression of florigen gene FT, and the regulation manner of FLC and FT provides a paradigm for control of developmental regulators through chromatin modifications (He, 2009). Currently, not so many data are available about that in rice, but molecular genetic studies indicated that rice flowering control also undergoes the complex chromatin modifications (Table 2).
ACTIVE CHROMATIN MODIFICATIONS AND RICE FLOWERING
S-Adenosyl-l-methionine is a universal methyl group donor involved in numerous transmethylation reactions, including histone methylation. Knockdown of rice S-Adenosyl-l-methionine synthetase (SAMS) 1, 2, and 3 greatly reduced the expression of Ehd1, Hd3a, RFT1 and led to a late flowering phenotype. Moreover, the histone H3K4me3 and symmetric DNA methylation at these genes was significantly reduced, suggesting an association between epigenetic modification and flowering in rice, but more research are required on this relationship (Li et al., 2011).
We have demonstrated that SDG724, a histone methyltransferase gene which belongs to SET domain family Class II (Ng et al., 2007), affected flowering time by mediating H3K36 methylation in rice. SDG724 loss-of-function mutant lvp1 showed a late flowering phenotype under both LD and SD, which was associated with the suppressed expression of RFT1 and Hd3a. Interestingly, only the chromosomal region of RFT1, but not Hd3a, reduced the level of H3K36me2/3 modifications which associated with the transcriptionally active chromatin state, although the two florigenic genes are closely linked in the genome and separated by only 11.5 kb (Sun et al., 2012). This similar regulated way in RFT1 is also found in a previous report that RFT1 expression can be promoted through another active histone modification H3K9 acetylation around the transcriptional start site of its chromatin in Hd3a-RNAi transgenic plants (Komiya et al., 2008). In conclusion, both of the two findings suggest an epigenetic regulation mechanism through RFT1. In addition, SDG724 also affects the histone modification state at OsMADS50 chromosomal region, thus all the results suggest a LD floral promotion pathway mediated by H3K36me2/3 deposition through OsMADS50-Ehd1-RFT1 pathways in rice (Sun et al., 2012).
Coincidentally, another member of Class II in SET domain family (Ng et al., 2007), SDG725, is also involved in promoting rice flowering through H3K36me2/3. In SDG725 knockdown plants, the expression levels of Ehd3, RID1, OsMADS50, OsMADS51, Ehd1, Hd3a, and RFT1 were all drastically reduced, but the Ghd7 expression was increased, under either SD or LD. Different from SDG724, SDG725 is required for deposition of H3K36me2/3 at more flowering gene loci, such as Ehd3, RID1, OsMADS50, Hd3a, and RFT1. Thus, SDG724 and SDG725 regulate both overlapped and specific flowering genes by mediating H3K36me2/3 deposition and promote rice flowering, which are different to the previously known function of these epigenetic marks in Arabidopsis flowering (Sui et al., 2012; Xu et al., 2008; Zhao et al., 2005).
Very recently, another homolog of SDG724, OsTrx1, which belongs to SET domain family Class III (Ng et al., 2007), might activate or maintain the active transcribed states of target genes, and was reported to delay flowering time under LD through Ghd7 pathway but not OsMADS50 and Hd1 pathways (Choi et al., 2014). Though expression of Ehd3 that functions upstream of Ghd7 is unchanged in ostrx1 mutants, it was proved that OsTrx1 could bind to Ehd3 in vitro. Further study showed that PHD motif of OsTrx1 could bind to native histone H3 and the C-terminal end of SET domain of OsTrx1 has histone H3 methyltransferase activity, thus OsTrx1 and Ehd3 tend to form a complex to methylate downstream genes, but further studies are needed to illuminate its function in detail (Choi et al., 2014).
REPRESSIVE CHROMATIN MODIFICATIONS AND RICE FLOWERING
Arabidopsis VILs (VIL, VERNALIZATION INSENSITIVE), VIN3, and VRN5 are components of PRC2 (Polycomb Repressive Complex 2), mediating the H3K27 trimethylation at the FLC locus to repress its expression and hence to induce flowering. In rice, a VIL homolog gene LC2/OsVIL3 is considered as a possible component of PRC2 complex, and lc2 mutants display late flowering along with the reduced expression of Hd1 and Hd3a under SD. Furthermore, consistent with the result that OsLF (Oryza sativaLate Flowering) directly repressed Hd1 expression (Zhao et al., 2011), LC2/OsVIL3 binds to the promoter region of OsLF and represses the OsLF expression via H3K27me3 methylation, thus eventually promotes flowering (Wang et al., 2012).
OsVIL2 may be another VILs member in rice PRC2 complex, and mutations in OsVIL2 cause late flowering under both SD and LD. Different from LC2/OsVIL3, the late flowering phenotype is associated with increased OsLFL1 and reduced Ehd1, Hd3a, RFT1 expression. Furthermore, OsVIL2 can bind to native histone H3 in vitro and is directly associated with OsLFL1 chromatin in vivo, and H3K27me3 is significantly reduced on OsLFL1 chromatin in osvil2 mutants compared to the wild type, indicating that OsVIL2 epigenetically represses OsLFL1 expression to promote flowering in rice. Besides, OsVIL2 can physically interact with OsEMF2b, which may be also a member of PRC2. Similar to osvil2, a null mutation of OsEMF2b caused late flowering by increasing OsLFL1 and decreasing Ehd1 expression (Wang et al., 2012; Yang et al., 2012).
In short, similar to Arabidopsis, LC2/OsVIL3, OsVIL2, and OsEMF2b may function together with PRC2 to induce flowering by affecting histone modification H3K27me3, but their target flowering genes are different, indicating that a diverse flowering pathway regulated by PRC2 in rice flowering.
CONCLUSION AND PERSPECTIVES
Heading date is an important agronomic trait that determining rice to grow in different regions and seasons. In last two decades, tremendous progress has been made by the study of QTLs and genes controlling rice flowering, which not only identified the nature of the mobile signal florigen, but also unveiled a complex genetic network that controls florigen in rice. Hd3a and RFT1, two florigens regulated respectively rice flowering under SD and LD, are mainly controlled through Hd1 and Ehd1 pathways. However, as mentioned in various T65 with lack of both, rice also develops some additional pathways that could induce rice flowering.
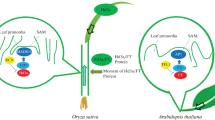
Histone modification is very important for defining transcriptional regulation expression, thus plays a fundamental role in plant growth and development, as well as responding to various environmental conditions. These modification marks are dynamically “written” and “erased”, and then specifically recognized by the “readers” and instruct specific biological process, such as flowering. Very recently, a large number of studies have revealed that various ‘active’ histone modifications, H3K4 methylation, H2B monoubiquitination, H3K36me2/me3, histone deacetylation, and ‘repressive’ chromatin modifications, H3K4 demethylation, H3K9 methylation, H3K27 methylation, histone arginine methylation, are involved in modulating FLC expression in Arabidopsis. Though the regulation of FLC expression via chromatin modification provides a paradigm in flowering gene expression, whether there exists a major flowering regulator such as FLC in rice is still unknown (Fig. 1). Rice possibly has some new routes in its flowering control. In rice, a number of studies revealed the difference in chromatin modification mechanism in the past two years, ‘active’ H3K36me2/3, H3K4me3, H3K9 acetylation and ‘repressive’ H3K27me3 modifications mediate flowering time through Hd1 and Ehd1 dependent pathways, and our finding about SDG724 also suggests a LD floral promotion pathway that could be mediated via an epigenetic regulation of florigen RFT1 itself. All these data suggest that the target flowering genes of chromatin modifications are dispersed in both conserved and unique flowering pathways in rice. Taken together, all the progress in rice, along with Arabidopsis, provides a complete evolutionarily comparative view of genetic and epigenetic flowering mechanisms in plants until now.
Furthermore, in rice, some histone modification participators tend to function under SD and LD, but others like to function mainly under SD or LD, thus unveiling of histone modification mechanism in rice flowering might set a solution to verify the relationships between particular histone modifications and photoperiod environments. On the other hand, as a LD plant, Arabidopsis flowering is accelerated by LD, but SD plant rice flowers earlier under SD than under LD, further study will be helpful to distinguish the function and evolutionary process of histone modification in various photoperiodic plants. Thus, it would be of great interest to identify more chromatin modification regulators and their target genes in rice flowering in future.
Abbreviations
- FLC :
-
FLOWERING LOCUS C
- FT :
-
FLOWERING LOCUS T
- H3K36:
-
Histone H3 lysine 36
- Hd:
-
heading date
- HMTase:
-
histone methyltransferase
- LD:
-
long day
- RFT1 :
-
RICE FLOWERING LOCUS T 1
- SAM:
-
shoot apical meristem
- SD:
-
short day
References
Andres F, Galbraith DW, Talon M, Domingo C (2009) Analysis of PHOTOPERIOD SENSITIVITY5 sheds light on the role of phytochromes in photoperiodic flowering in rice. Plant Physiol 151:681–690
Brambilla V, Fornara F (2013) Molecular control of flowering in response to day length in rice. J Integr Plant Biol 55:410–418
Buckler ES, Holland JB, Bradbury PJ, Acharya CB, Brown PJ, Browne C, Ersoz E, Flint-Garcia S, Garcia A, Glaubitz JC, Goodman MM, Harjes C, Guill K, Kroon DE, Larsson S, Lepak NK, Li H, Mitchell SE, Pressoir G, Peiffer JA, Rosas MO, Rocheford TR, Romay MC, Romero S, Salvo S, Sanchez Villeda H, da Silva HS, Sun Q, Tian F, Upadyayula N, Ware D, Yates H, Yu J, Zhang Z, Kresovich S, McMullen MD (2009) The genetic architecture of maize flowering time. Science 325:714–718
Cajlachjan MC (1937) Concerning the hormonal nature of plant development processes. Compt Rend Acad Sci URSS 16:227–230
Chardon F, Damerval C (2005) Phylogenomic analysis of the PEBP gene family in cereals. J Mol Evol 61:579–590
Choi SC, Lee S, Kim S-R, Lee Y-S, Liu C, Cao X, An G (2014) Trithorax group protein OsTrx1 controls flowering time in rice via interaction with Ehd3. Plant Physiol 164(3):1326–1337
Colasanti J, Yuan Z, Sundaresan V (1998) The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 93:593–603
Colasanti J, Tremblay R, Wong AY, Coneva V, Kozaki A, Mable BK (2006) The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genomics 7:158
Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316:1030–1033
Dai C, Xue HW (2010) Rice early flowering1, a CKI, phosphorylates DELLA protein SLR1 to negatively regulate gibberellin signalling. EMBO J 29:1916–1927
Dai X, Ding Y, Tan L, Fu Y, Liu F, Zhu Z, Sun X, Sun X, Gu P, Cai H, Sun C (2012) LHD1, an allele of DTH8/Ghd8, controls late heading date in common wild rice (Oryza rufipogon). J Integr Plant Biol 54:790–799
Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev 18:926–936
Ebana K, Shibaya T, Wu J, Matsubara K, Kanamori H, Yamane H, Yamanouchi U, Mizubayashi T, Kono I, Shomura A, Ito S, Ando T, Hori K, Matsumoto T, Yano M (2011) Uncovering of major genetic factors generating naturally occurring variation in heading date among Asian rice cultivars. Theor Appl Genet 122:1199–1210
Fujino K, Yamanouchi U, Yano M (2013) Roles of the Hd5 gene controlling heading date for adaptation to the northern limits of rice cultivation. Theor Appl Genet 126:611–618
Gao H, Zheng XM, Fei G, Chen J, Jin M, Ren Y, Wu W, Zhou K, Sheng P, Zhou F, Jiang L, Wang J, Zhang X, Guo X, Wang JL, Cheng Z, Wu C, Wang H, Wan JM (2013) Ehd4 encodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice. PLoS Genet 9:e1003281
Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422:719–722
He Y (2009) Control of the transition to flowering by chromatin modifications. Mol Plant 2:554–564
Hori K, Kataoka T, Miura K, Yamaguchi M, Saka N, Nakahara T, Sunohara Y, Ebana K, Yano M (2012) Variation in heading date conceals quantitative trait loci for other traits of importance in breeding selection of rice. Breed Sci 62:223–234
Hori K, Ogiso-Tanaka E, Matsubara K, Yamanouchi U, Ebana K, Yano M (2013) Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day-length response. Plant J 76:36–46
Ishikawa R, Aoki M, Kurotani K, Yokoi S, Shinomura T, Takano M, Shimamoto K (2011) Phytochrome B regulates Heading date 1 (Hd1)-mediated expression of rice florigen Hd3a and critical day length in rice. Mol Genet Genomics 285:461–470
Itoh H, Izawa T (2013) The coincidence of critical day length recognition for florigen gene expression and floral transition under long-day conditions in rice. Mol Plant 6:635–649
Itoh H, Nonoue Y, Yano M, Izawa T (2010) A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat Genet 42:635–638
Izawa T (2007) Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J Exp Bot 58:3091–3097
Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K (2002) Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev 16:2006–2020
Kim SL, Lee S, Kim HJ, Nam HG, An G (2007) OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a. Plant Physiol 145:1484–1494
Kim SK, Yun CH, Lee JH, Jang YH, Park HY, Kim JK (2008) OsCO3, a CONSTANS-LIKE gene, controls flowering by negatively regulating the expression of FT-like genes under SD conditions in rice. Planta 228:355–365
Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43:1096–1105
Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135:767–774
Komiya R, Yokoi S, Shimamoto K (2009) A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136:3443–3450
Koo BH, Yoo SC, Park JW, Kwon CT, Lee BD, An G, Zhang Z, Li J, Li Z, Paek NC (2013) Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol Plant 6:1877–1888
Kwon CT, Yoo SC, Koo BH, Cho SH, Park JW, Zhang Z, Li J, Li Z, Paek NC (2013) Natural variation in Early flowering1 contributes to early flowering in japonica rice under long days. Plant Cell Environ 37:101–112
Lee S, Kim J, Han JJ, Han MJ, An G (2004) Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. Plant J 38:754–764
Lee YS, Jeong DH, Lee DY, Yi J, Ryu CH, Kim SL, Jeong HJ, Choi SC, Jin P, Yang J, Cho LH, Choi H, An G (2010) OsCOL4 is a constitutive flowering repressor upstream of Ehd1 and downstream of OsphyB. Plant J 63:18–30
Li D, Yang C, Li X, Gan Q, Zhao X, Zhu L (2009) Functional characterization of rice OsDof12. Planta 229:1159–1169
Li W, Han Y, Tao F, Chong K (2011) Knockdown of SAMS genes encoding S-adenosyl-l-methionine synthetases causes methylation alterations of DNAs and histones and leads to late flowering in rice. J Plant Physiol 168:1837–1843
Lin S, Sasaki T, Yano M (1998) Mapping quantitative trait loci controlling seed dormancy and heading date in rice, Oryza sativa L., using backcross inbred lines. Theor Appl Genet 96:997–1003
Lin HX, Yamamoto T, Sasaki T, Yano M (2000) Characterization and detection of epistatic interactions of 3 QTLs, Hd1, Hd2, and Hd3, controlling heading date in rice using nearly isogenic lines. Theor Appl Genet 101:1021–1028
Lin H, Liang Z-W, Sasaki T, Yano M (2003) Fine mapping and characterization of quantitative trait loci Hd4 and Hd5 controlling heading date in rice. Breed Sci 53:51–59
Liu C, Lu F, Cui X, Cao X (2010) Histone methylation in higher plants. Annu Rev Plant Biol 61:395–420
Liu T, Liu H, Zhang H, Xing Y (2013) Validation and characterization of Ghd7.1, a major quantitative trait locus with pleiotropic effects on spikelets per panicle, plant height, and heading date in rice (Oryza sativa L.). J Integr Plant Biol 55:917–927
Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, Yano M (2008) Ehd2, a rice ortholog of the maize ID1 gene, promotes flowering by upregulating Ehd1. Plant Physiol 148:1425–1435
Matsubara K, Yamanouchi U, Nonoue Y, Sugimoto K, Wang ZX, Minobe Y, Yano M (2011) Ehd3, encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering. Plant J 66:603–612
Matsubara K, Ogiso-Tanaka E, Hori K, Ebana K, Ando T, Yano M (2012) Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol 53:709–716
Ng DW, Wang T, Chandrasekharan MB, Aramayo R, Kertbundit S, Hall TC (2007) Plant SET domain-containing proteins: structure, function and regulation. Biochim Biophys Acta 1769:316–329
Nonoue Y, Fujino K, Hirayama Y, Yamanouchi U, Lin SY, Yano M (2008) Detection of quantitative trait loci controlling extremely early heading in rice. Theor Appl Genet 116:715–722
Ogiso E, Takahashi Y, Sasaki T, Yano M, Izawa T (2010) The role of casein kinase II in flowering time regulation has diversified during evolution. Plant Physiol 152:808–820
Park SJ, Kim SL, Lee S, Je BI, Piao HL, Park SH, Kim CM, Ryu CH, Park SH, Xuan YH, Colasanti J, An G, Han CD (2008) Rice Indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J 56:1018–1029
Peng LT, Shi ZY, Li L, Shen GZ, Zhang JL (2007) Ectopic expression of OsLFL1 in rice represses Ehd1 by binding on its promoter. Biochem Biophys Res Commun 360:251–256
Peng LT, Shi ZY, Li L, Shen GZ, Zhang JL (2008) Overexpression of transcription factor OsLFL1 delays flowering time in Oryza sativa. J Plant Physiol 165:876–885
Ryu CH, Lee S, Cho LH, Kim SL, Lee YS, Choi SC, Jeong HJ, Yi J, Park SJ, Han CD, An G (2009) OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant Cell Environ 32:1412–1427
Saito H, Ogiso-Tanaka E, Okumoto Y, Yoshitake Y, Izumi H, Yokoo T, Matsubara K, Hori K, Yano M, Inoue H, Tanisaka T (2012) Ef7 encodes an ELF3-like protein and promotes rice flowering by negatively regulating the floral repressor gene Ghd7 under both short- and long-day conditions. Plant Cell Physiol 53:717–728
Salome PA, Bomblies K, Laitinen RA, Yant L, Mott R, Weigel D (2011) Genetic architecture of flowering-time variation in Arabidopsis thaliana. Genetics 188:421–433
Shibaya T, Nonoue Y, Ono N, Yamanouchi U, Hori K, Yano M (2011) Genetic interactions involved in the inhibition of heading by heading date QTL, Hd2 in rice under long-day conditions. Theor Appl Genet 123:1133–1143
Sui P, Shi J, Gao X, Shen WH, Dong A (2012) H3K36 methylation is involved in promoting rice flowering. Mol Plant 6:975–977
Sun C, Fang J, Zhao T, Xu B, Zhang F, Liu L, Tang J, Zhang G, Deng X, Chen F, Qian Q, Cao X, Chu C (2012) The histone methyltransferase SDG724 mediates H3K36me2/3 deposition at MADS50 and RFT1 and promotes flowering in rice. Plant Cell 24:3235–3247
Takahashi Y, Shimamoto K (2011) Heading date 1 (Hd1), an ortholog of Arabidopsis CONSTANS, is a possible target of human selection during domestication to diversify flowering times of cultivated rice. Genes Genet Syst 86:175–182
Takahashi Y, Shomura A, Sasaki T, Yano M (2001) Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the alpha subunit of protein kinase CK2. Proc Natl Acad Sci USA 98:7922–7927
Takahashi Y, Teshima KM, Yokoi S, Innan H, Shimamoto K (2009) Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc Natl Acad Sci USA 106:4555–4560
Takimoto A, Ikeda K (1961) Effect of twilight on photoperiodic induction in some short day plants. Plant Cell Physiol 2:213–229
Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316:1033–1036
Tsuji H, Taoka K, Shimamoto K (2011) Regulation of flowering in rice: two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol 14:45–52
Tsuji H, Taoka K, Shimamoto K (2013) Florigen in rice: complex gene network for florigen transcription, florigen activation complex, and multiple functions. Curr Opin Plant Biol 16:228–235
Wang J, Hu J, Qian Q, Xue HW (2012) LC2 and OsVIL2 promote rice flowering by photoperoid-induced epigenetic silencing of OsLF. Mol Plant 6:514–527
Wei X, Xu J, Guo H, Jiang L, Chen S, Yu C, Zhou Z, Hu P, Zhai H, Wan J (2010) DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol 153:1747–1758
Wu C, You C, Li C, Long T, Chen G, Byrne ME, Zhang Q (2008) RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc Natl Acad Sci USA 105:12915–12920
Wu W, Zheng XM, Lu G, Zhong Z, Gao H, Chen L, Wu C, Wang HJ, Wang Q, Zhou K, Wang JL, Wu F, Zhang X, Guo X, Cheng Z, Lei C, Lin Q, Jiang L, Wang H, Ge S, Wan J (2013) Association of functional nucleotide polymorphisms at DTH2 with the northward expansion of rice cultivation in Asia. Proc Natl Acad Sci USA 110:2775–2780
Xu L, Zhao Z, Dong A, Soubigou-Taconnat L, Renou JP, Steinmetz A, Shen WH (2008) Di- and tri- but not monomethylation on histone H3 lysine 36 marks active transcription of genes involved in flowering time regulation and other processes in Arabidopsis thaliana. Mol Cell Biol 28:1348–1360
Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, Zhang Q (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yamamoto T, Lin H, Sasaki T, Yano M (2000) Identification of heading date quantitative trait locus Hd6 and characterization of its epistatic interactions with Hd2 in rice using advanced backcross progeny. Genetics 154:885–891
Yan WH, Wang P, Chen HX, Zhou HJ, Li QP, Wang CR, Ding ZH, Zhang YS, Yu SB, Xing YZ, Zhang QF (2011) A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant 4:319–330
Yan W, Liu H, Zhou X, Li Q, Zhang J, Lu L, Liu T, Zhang C, Zhang Z, Shen G, Yao W, Chen H, Yu S, Xie W, Xing Y (2013) Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice. Cell Res 23:969–971
Yang J, Lee S, Hang R, Kim SR, Lee YS, Cao X, Amasino R, An G (2012) OsVIL2 functions with PRC2 to induce flowering by repressing OsLFL1 in rice. Plant J 73:566–578
Yang Y, Peng Q, Chen GX, Li XH, Wu CY (2013) OsELF3 is involved in circadian clock regulation for promoting flowering under long-day conditions in rice. Mol Plant 6:202–215
Yano M, Harushima Y, Nagamura Y, Kurata N, Minobe Y, Sasaki T (1997) Identification of quantitative trait loci controlling heading date in rice using a high-density linkage map. Theor Appl Genet 95:1025–1032
Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12:2473–2484
Zhao Z, Yu Y, Meyer D, Wu C, Shen WH (2005) Prevention of early flowering by expression of FLOWERING LOCUS C requires methylation of histone H3 K36. Nat Cell Biol 7:1256–1260
Zhao XL, Shi ZY, Peng LT, Shen GZ, Zhang JL (2011) An atypical HLH protein OsLF in rice regulates flowering time and interacts with OsPIL13 and OsPIL15. Nat Biotechnol 28:788–797
Zhao J, Huang X, Ouyang X, Chen W, Du A, Zhu L, Wang S, Deng XW, Li S (2012) OsELF3-1, an ortholog of Arabidopsis early flowering 3, regulates rice circadian rhythm and photoperiodic flowering. PLoS ONE 7:e43705
ACKNOWLEDGMENTS
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 31371602 and 91335107) and Specialized Research Fund for the Doctoral Program of Higher Education (20125103120008).
COMPLIANCE WITH ETHICS GUIDELINES
Changhui Sun, Dan Chen, Jun Fang, Pingrong Wang, Xiaojian Deng, and Chengcai Chu declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sun, C., Chen, D., Fang, J. et al. Understanding the genetic and epigenetic architecture in complex network of rice flowering pathways. Protein Cell 5, 889–898 (2014). https://doi.org/10.1007/s13238-014-0068-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13238-014-0068-6