Abstract
Context
Intravaginal placement of misoprostol has been used extensively to terminate second trimester pregnancies. Intracervical misoprostol is an alternative method of termination of pregnancy for women in this period of gestation.
Objective
To assess the efficacy and safety of combined intracervical and intravaginal misoprostol in the management of mid-trimester medical termination of pregnancy and to compare it with intravaginal misoprostol.
Materials and Methods
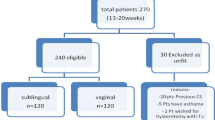
In this IRB approved prospective study, twenty-two women (mean age 25.4 ± 3.2 years, range 23–32 years; mean BMI 22.3 ± 3.4 kg/m2; mean parity 2.1 ± 1.4, average gestational age 17.9 ± 2.4 weeks) underwent second trimester termination of pregnancy at our institution. Patient cohort was randomized into two treatment protocols depending on the drug used and route of administration. Induction-abortion interval, need for surgical evacuation, completeness of abortion and side effects if any were documented.
Results
Mean induction-abortion interval for intravaginal group and combination group was comparable (t = 7.9 ± 1.8 and 6.5 ± 3.5 h, respectively). Three patients required surgical evacuation for incomplete abortion (n = 2 after vaginal misoprostol and one after intracervical–intravaginal misoprostol). Number of patients aborting within 6 h was more in the intracervical–intravaginal group (36.3 %). Patients with intracervical misoprostol complained of abdominal pain more often than those in other groups. Excessive bleeding and uterine rupture was not seen in any patient.
Conclusion
Intracervical misoprostol is an effective method of medical treatment of second trimester pregnancy failure. Its short induction to abortion interval and acceptable safety profile makes induction via the cervical route acceptable for second trimester abortion.


Similar content being viewed by others
References
Berghella V, Airoldi J, O’Neill AM, et al. Misoprostol for second trimester pregnancy termination in women with prior caesarean: a systematic review. BJOG. 2009;116:1151.
Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304.
Ramsey PS, Savage K, Lincoln T, et al. Vaginal misoprostol versus concentrated oxytocin and vaginal PGE2 for second-trimester labor induction. Obstet Gynecol. 2004;104:138.
Webster D, Penney GC, Templeton A. A comparison of 600 and 200 mg mifepristone prior to second trimester abortion with the prostaglandin misoprostol. Br J Obstet Gynaecol. 1996;103:706–9.
Ashok PW, Templeton A, Wagaarachchi PT, et al. Mid trimester medical termination of pregnancy: a review of 1002 consecutive cases. Contraception. 2004;69(1):51–8.
Rouzi AA, Almansouri N, Sahly N, et al. Efficacy of intra-cervical misoprostol in the management of early pregnancy failure. Sci Rep. 2014;4:7182.
Ghorab MN, El Helw BA, Nabil M. Second-trimester termination of pregnancy by extra-amniotic prostaglandin F2alpha or endocervical misoprostol: a comparative study. Acta Obstet Gynecol Scand. 1998;77(4):429–32.
Paz B, Ohel G, Tal T, et al. Second trimester abortion by laminaria followed by vaginal misoprostol or intrauterine prostaglandin F2alpha: a randomized trial. Contraception. 2002;65(6):411–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors have no conflict of interest.
Ethical Standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5).
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Gaurav S Desai, Registrar; Abhishek Chandavarkar, Lecturer; Sriram Gopal, Professor; Ganpat Sawant, Professor; Shyam V Desai, Professor.
Rights and permissions
About this article
Cite this article
Desai, G.S., Chandavarkar, A., Gopal, S. et al. Second Trimester Medical Termination of Pregnancy with Combined Intracervical and Intravaginal Misoprostol: Comparative Analysis with Intravaginal Misoprostol—A Pilot Study. J Obstet Gynecol India 66 (Suppl 1), 157–160 (2016). https://doi.org/10.1007/s13224-015-0827-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-015-0827-1