Abstract
The vascular system, essential for human physiology, is vital for transporting nutrients, oxygen, and waste. Since vascular structures are involved in various disease pathogeneses and exhibit different morphologies depending on the organ, researchers have endeavored to develop organ-specific vascular models. While animal models possess sophisticated vascular morphologies, they exhibit significant discrepancies from human tissues due to species differences, which limits their applicability. To overcome the limitations arising from these discrepancies and the oversimplification of 2D dish cultures, microphysiological systems (MPS) have emerged as a promising alternative. These systems more accurately mimic the human microenvironment by incorporating cell interactions, physical stimuli, and extracellular matrix components, thus facilitating enhanced tissue differentiation and functionality. Importantly, MPS often utilize human-derived cells, greatly reducing disparities between model and patient responses. This review focuses on recent advancements in MPS, particularly in modeling the human organ-specific vascular system, and discusses their potential in biological adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The vascular system, comprising a vast network of arteries, veins, and capillaries, is essential to the proper functioning of the human body. These vessels ensure the efficient transport of oxygen, nutrients, hormones, immune cells, and even drugs to every tissue and organ while simultaneously facilitating the removal of waste products. They serve not only as simple nourishing components but are also specialized across various organs and tissues to fulfill unique roles. For instance, within similar microvessel systems, the blood–brain barrier of the neurovascular system is highly impermeable to block cytotoxic materials, whereas the tumor microvascular system is usually leaky [1, 2]. To understand the various features of vessel system, researchers have sought sophisticated vascular platforms that possess the morphological and physiological characteristics of each respective tissue.
Animal models have been conventionally employed as alternatives for 2D dish culture models to simulate the intricate morphologies of tissues. They have consistently revealed insights, especially arising from complex interactions among fully differentiated cells, hardly observed in dish culture models. However, significant discrepancies have persisted between animal models and human tissues, originating from species differences, even with the rigorous selection of model organisms. For example, while mice have been frequently used in retinal studies, their retinal thickness and developmental trajectories significantly differ from those of humans, leading to distinct responses [3]. Considering the reactions to biochemical agents and diverse stimuli, the discrepancy between animals and human becomes amplified [4]. Furthermore, compared to straightforward dish culture methods, manipulation in animal models is considerably more challenging, as it involves dealing with the entire living organism, not just the target organ.
To compensate for the limitations associated with the oversimplification of 2D dish culture models and the complexity and discrepancies of animal models, microphysiological systems have been widely applied. These systems aim to replicate the microphysiological environment, encompassing cell interactions, physical stimuli, biochemical gradients, and extracellular matrix (ECM) surroundings, thereby improving tissue differentiation and functionality from 2D models. Also, microphysiological systems (MPS) are specifically designed with a focus on the target tissue and tailored for the experimental approach, resulting in easier manipulation and maintenance compared to animal models [5, 6]. Moreover, since these systems are often constructed using human-origin cells or even patient-derived cells, the discrepancies between the model and patient responses are substantially reduced. In this review, we summarize the various models of vascular systems with particular emphasis on the recent advances of microphysiological systems, and categorize them by organs and fabrication method.
2 Conventional Experimental Platform for Blood Vessel-Related Diseases and Drug Screening
2.1 In Vivo Models
Historically, animal models have been indispensable for disease modeling, drug efficacy, and toxicity testing. These models' strength lies in their anatomical and physiological similarities to humans, especially in terms of vasculature and organ systems [7]. They provide a comprehensive perspective on the entire organism and allow for versatile modeling of conditions through genetic manipulation [8]. Despite these advantages, animal models demand considerable time and financial resources, raise ethical issues, and exhibit disparities in size and genetics compared to humans [7]. Specifically, certain disease modeling methods in animals, like gene knockouts, might not accurately resemble human conditions. For instance, while genetic manipulations are able to induce atherosclerosis in mice, the plaque ruptures do not occur, unlike human cases [9]. Similarly, there are also discrepancies in retinal tissue, such as the thickness of Bruch's membrane and the ratio of binucleated RPE [10]. As a result, drugs considered safe in animal trials occasionally demonstrate toxicity in humans; indeed, nearly half of the 578 drugs withdrawn or discontinued in Europe and the US have shown toxicity to humans [11], whereas penicillin and aspirin, which are non-toxic and widely applied to humans, exhibit toxicity in certain animal models [11]. Due to platform inherent limitations of the animal model, including genomic disparities, we categorize it within the conventional model framework, in spite of its significant enhancements and valuable achievements. Also, the current situation, that species-specific differences cannot be minimized through additional experimental approaches, supports this categorization. Alternatively, advanced models discussed in the subsequent section have addressed some of these limitations and are progressively overcoming remaining challenges through various improvements.
2.2 In Vitro Culture Models
To minimize the genomic differences between human and animal models, researchers have cultured human-derived cells. The two-dimensional (2D) in vitro model is the oldest method and has long been applied in various studies, including pathogenesis unveiling and drug screening [12]. Typically, these 2D models were designed as a monolayer of specific cell types on Petri dishes or well plates. These models offer simplicity and high-throughput reproducibility at a relatively low experimental cost [13]. Nevertheless, 2D models have inherent drawbacks, including inaccurate oxygen and nutrient distribution and, more importantly, the lack of interactions between cells in the 3D extracellular matrix [14].
Three-dimensional (3D) compartmentalized in vitro models encompass membrane models, notably represented by the Transwell system. The Transwell system easily facilitates the co-culture of multiple cell types in separated upper and lower compartments, divided by a porous membrane. This setup has been often applied to measure cell migration or barrier function. However, these 3D membrane models still exhibit certain limitations inherent in 2D in vitro models; the cells are cultured as a 2D monolayer on a rigid substrate rather than within a 3D extracellular matrix [15]. Particularly, the endothelium cultured on Transwell shows a considerably higher permeability compared to in vivo blood vessels [15, 16].
Another method is 3D cell culture within various hydrogels, which serve as scaffolds for 3D culture and promote cell differentiation, similar to the ECM surrounding cells in vivo. In this model, researchers have utilized the native extracellular matrix (ECM) to enhance cell differentiation and have also employed synthetic ECM to apply a range of mechanical and structural cues [17]. However, this technique is not ideally suited for vascular research and associated diseases, as manipulating cellular positioning and perfusion is challenging without incorporating a microfluidic device.
Through the basic 2D dish culture models to sophisticated 3D cell culture models that utilize novel ECM, in vitro culture models have addressed genomic disparities of animal models and provided valuable insights. These achievements in cell culture techniques and ECM design are now being incorporated into following advanced models. However, like animal models, these systems now encounter the limitations, particularly in applying additional experimental approaches to enhance in vivo similarity and differentiation levels. For instance, the application of physical stimuli such as flow, stress, and electric fields, which is essential for cell differentiation, remains challenging in in vitro culture models without MPS integration. Given these considerations, we categorize both in vitro culture models and animal models within the conventional model framework.
3 Advanced In Vitro Models for Vascular Study
3.1 Organoid Models
In this paper, we have defined advanced models as novel systems that overcome the traditional challenges of conventional models by incorporating recently highlighted stimuli and experimental methods, and which still have room for extension. As an advanced 3D model, organoids are able to emulate organ functionalities more properly, using variety stimulation and manipulation. They are derived from self-assembling pluripotent cells, or adult stem cells [8]. Organoids vary from simple epithelial constructs to sophisticated structures that closely resemble in vivo organs. For instance, in case of in vitro microvessel construction, 3D hydrogel culture enables the formation of a microvascular network with in vivo similar aspects, including permeability, comparing to traditional 2D transwell models [16]. Furthermore, the blood vessel model produced by organoid systems can generate not only the endothelial cell (EC) vessel network but also the surrounding tissues and supportive cells, so that it can resemble in vivo tissue reactions properly [21]. They have provided invaluable observations in fundamental biological investigations and potential pharmaceutical evaluations (Fig. 1A) [8].
Practically, organoids have been known to be challenging to vascularize due to various reasons, including their size, the difficult spatial regulation of tissue, and the challenge of co-inducing endothelial cell (EC) supportive cells and organ-specific cells. However, recently several organoid studies have focused on modeling vasculature. Wimmer et al. constructed a self-organizing 3D vascular network organoid from pluripotent stem cells, which was more sophisticated and enhanced than the conventional transwell method [21]. In this method, cells were properly differentiated, and vessels were enveloped by pericytes and a basement membrane, mimicking in vivo conditions. The organoid was fully functional and could be transplanted into mice, forming a stable and perfusable vascular tree, including arteries and arterioles. Under diabetic conditions, it exhibited in vivo-like reactions, such as basement membrane thickening. Researchers also constructed specific organs supported by vascular tissues. Lewis-Israeli et al. designed a self-assembling human heart organoid with self-nourishing blood vessels [22]. Stem cells were differentiated into various cardiac cells, including the essential cardiomyocytes and endothelial cells. The endothelium formed vascular lumens that penetrated the myocardium and assembled into coronary vessels.
To apply the physical stimuli like perfusion, additional housing or chambers, such as MPS, are often adapted. Homan et al. constructed a kidney organoid with a perfusable vascular network, employing flow within the organoid [23]. This fluidic stimulus not only advanced the vascular network but also enhanced kidney-specific differentiation, such as podocyte maturity and cellular polarity. In this study, a millifluidic chip was used to introduce flow conditions.
3.2 Bioprinted Models
The last decade has witnessed significant contributions to vascular tissue engineering with the rise of 3D bioprinting technology (Fig. 1B). This cutting-edge technology involves the intricate process of printing cell-laden hydrogels and polymeric scaffolds. Blood vessels in the human body vary widely in size, ranging from microvessels with internal diameters less than 1mm to larger vessels with internal diameters exceeding 6 mm [24,25,26]. As the field is advancing with novel bioinks and stem cells, to simulate natural diversity of blood vessel, for creation of vascular models with variety of diameters and patterns through the precise compartmentalization of vascular cell types, such as endothelial cells and smooth muscle cells, 3D bioprinting technology is making it possible to fabricate the vascular models ranging from micrometer to millimeter size with a high precision at micron scale [27, 28].
For bioprinting vascular models with variety of sizes and patterns, various bioprinting technologies, including inkjet [29] and laser-assisted bioprinting [30], as well as extrusion-based printing [31], have been used. Bioprinting technology has achieved a minimum internal diameter of approximately 400 microns, while the largest reported diameter is around 6 mm [32,33,34]. Notably, the simultaneous printing of diverse cell types and polymer systems is made more accessible through the use of integrated multi-nozzle extrusion-based 3D bioprinters [35]. In one of the initial efforts, Xu et al. successfully generated 3D tubular structures, including both straight and zigzag configurations, with an approximate diameter of 3 mm by employing the inkjet bioprinting technique [29]. This effort paved the way for the possibility of bioprinting blood vessels with intricate geometries and structures. Using laser-assisted bioprinting method that involves the precise deposition of living cells directly from cell culture suspensions at specific locations, achieving a remarkable resolution of ± 5 μm on 2D or 3D substrates [36], Xiong et al. [30] achieved the fabrication of straight and Y-shaped hollow channels using cell-laden bioink, entirely without the need for support structures. This successful demonstration underscores the feasibility of this technique for creating overhanging structures.
For mimicking the native blood vessel’s anatomy, micro-extrusion based multi-axial nozzle system has been widely explored [35, 37,38,39]. This method allows straightforward printing of cell-laden bioinks in accordance with the natural structure of blood vessels, with desired diameter. In 2016, Jia et al. made a multi-axial nozzle extrusion bioprinting system and achieved the successful production of various cell-laden vascular constructs using hydrogel blend comprising gelatin methacryloyl (GelMA), alginate, and poly(-ethylene glycol)-tetra-acrylate (PEGTA) and confirmed the biocompatibility of bio-printed channels [33]. To closely mimic the native vascular system, Gao et al. employed a multi-nozzle system in using a bioink derived from porcine aortic tissue [35]. Using tissue-specific bioink, Gao et al. successfully printed a multi-layered blood vessel model that featured a smooth muscle layer enveloping the endothelium [40]. This study demonstrated excellent patency, the retention of a well-formed endothelium, the maturation of smooth muscle, and successful integration with host tissues as well. Wu et al. used this multi-axial nozzle system to 3D bioprint a micro-sized vascular channel that was both biocompatible and mechanically robust [41]. This was achieved by utilizing an unique formulation of bioink comprised of GelMA, methacrylated‐silk (SilkMA), and alginate. The resulting composite bioink facilitated the swift endothelialization of the channel, while also imparting it with excellent mechanical strength. This multi-axial nozzle bioprinting technology is now being used for advanced disease modeling of vascular pathologies. Gao et al. successfully demonstrated the fabrication of a novel atherosclerotic model utilizing this technology [35]. This triple-layered atherosclerotic blood vessel model has significantly expanded the horizons of 3D bioprinting technologies in the realm of biomedical applications, particularly in investigating the pathophysiology of cardiovascular diseases and for the development of drugs aimed at more effective therapies.
4 Microphysiological Systems as Vascular Models
To address the shortcomings of conventional in vitro models and mitigate the drawbacks of animal models, microphysiological systems (MPS) are garnering interest as a promising in vitro alternative. These systems, also known as organ-on-a-chip, can recreate three-dimensional tissues and blood vessels by integrating various types of human-derived cells into a spatially compartmentalized microfluidic chip with ECM and diverse mechanical properties (Fig. 1C). Since MPS incorporate an unprecedented variety of stimuli and variables to achieve enhanced human-similarity, they can be classified as advanced models. For instance, it allows to replicate the physiological concentration gradients of cytokines, chemicals, and drugs through three-dimensional diffusion [42]. Also, it can emulate physiological physical stimuli on organs and blood vessels, such as perfusion and stretching, through a microfluidic system [43, 44]. Some of these stimuli could be applied in conventional models, but we believe MPS have enhanced the ease of this process. Furthermore, unlike animal models, MPS can incorporate a variety of cell types, including human cells, genetically engineered cells, and even patients derived sample to resemble the subject-specific features. By combining the advances of the MPS, researchers can design in vitro platforms that closely resemble physiological conditions, potentially mitigating the constraints of the conventional models.
4.1 Categorization of Vascular MPS by Geometry
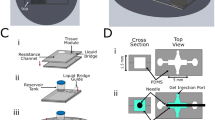
The vascular models using MPS can be categorized into two main groups based on their vascular structure. These groups can further be divided into subgroups based on their fabrication methods (Fig. 2). The first group is referred to as the “endothelium covering a channel” group, in which the shape of the blood vessel is determined by the configuration of the channel within the device. This group can be further subdivided into three subgroups. First, in case of "Multi-layered", the device is constructed with multiple layers of channels separated by semi-permeable membranes, similar to Transwell system [45]. These membranes are typically made of polymers and are coated with ECM proteins such as fibronectin. Endothelial cells are injected into one of these channels, while another cell types, such as smooth muscle cells for an artery model, are injected into the remaining channels. The second subgroup within this category is the "Parallel-patterned" model, which consists of parallel channels featuring micro-pillars or guide-edges [46]. Those structures provide a 100 μm scale gap, thereby establishing surface tension on the interface of injected ECM and compartmentalizing the channels. One channel is filled with hydrogel to compartmentalize, and endothelium are injected into the adjacent channel to form a vascular structure with ECM wall. The last subgroup is the "Cylindrical blood vessel" model, which the channel comprises a cavity within the ECM chamber [47, 48]. This cavity is created by the process, injecting hydrogel into the device within the cylindrical sacrificial mold, typically a needle. After the gelation, the mold is removed, and endothelial cells are injected into the cylindrical cavity.
In vitro vessel models with tumor using advanced in vitro system. (A) Incorporating the mesodermal progenitor cells, vascularized organoid was produced and the vessel network was validated by observing vessel-specific markers. (B) Using coaxial nozzle bioprinting method, the brain artery metastasis environment was simulated. (C) By co-seeding tumor spheroids in the microvascular network of MPS, the tumor microvascular niche was resembled. Figures are reprinted with permission from reference [18,19,20]. BEC brain endothelial cell, BPC brain pericyte, NPC neural progenitor cell, CTC circulating tumor cell, TC tumor cell, FB fibroblast
The second main group is "microvascular networks (μVNs)." This group can also be divided into two subgroups. The first subgroup is referred to as "self-organized μVNs," which fabricated by injection of endothelium-laden ECM, typically fibrinogen [49]. Inside the 3D fibrin gel, cells proliferate and automatically assemble into microvascular networks. To promote this process, extraneous growth factors can be added, or stromal cells can be co-seeded. The other subgroup within this category is "angiogenesis μVNs." In this case, endothelial cells are placed on the lateral side of the fibrin gel, and angiogenesis is also induced by growth factor or fibroblasts [45, 50].
While all types of vascular models using MPS can accommodate the abovementioned advantages of MPS, the different groups are used depending on the main aim or requirement of each studies. The first group models are usually utilized to generate vascular structures that are typically in the order of hundreds of micrometer or larger and also when compartmentalization of different cell types are needed. On the other hand, the microvascular network groups are used for generating microvasculature, representing a more physiologically relevant approach, as these vasculature structures form naturally through self-organization or angiogenesis.
Classification of microphysiological system based vascular models. The vascular system within the MPS was created by coating the interior of the cavity formed through compartmentalization (A1, A2) or using a sacrificial mold (A3). By harnessing the intrinsic abilities of ECs, microvessels can also be constructed through vascularization (B1) and angiogenesis(B2), involving ECM degradation. Figures are reprinted with permission from reference [45, 46, 48, 49, 51]
Organ-specific vascular models based on microphysiological system. (A) Coronary artery was reproduced by adapting the coaxial bioprinting method using ECs and SMCs. (B) Inducing vascularization of ECs, with neurovascular cells (PCs and ACs) resulted in a highly non-permeable microvessel network, similar to the in vivo blood-brain barrier. (C) By co-seeding the RPE monolayer on the EC microvessel network, the outer blood-retinal barrier was constructed and resembled the retina's neovascularization through hypoxic stress. (D) To replicate the glomerulus barrier, podocytes and ECs were cultured on the GBM layer. Membranous nephropathy was also observed under conditions involving patient-derived serum. Figures are reprinted with permission from reference [60, 75, 86, 95]. EC endothelial cells, PC pericyte, AC astrocyte, RPE retinal pigment epithelial cell, GBM glomerular basement membrane)
4.2 Organ-Specific Vascular MPS
4.2.1 Artery Models
Recent studies have achieved the development of "Artery-on-a-chip" systems by co-culturing endothelial cells and smooth muscle cells within microphysiological platforms (Fig. 3A). Some researchers adopted a “Multi-layered” platform and cultured endothelial and smooth muscle cells in each channel [52, 53]. Youn et al. rather than employing a PDMS membrane, devised a membrane fabricated by silk fibroin (SF) and polycaprolactone (PCL) to enhance the structural and physiological resemblance to in vivo arteries [54]. These models offer the benefit of ease in controlling physical stimuli and cellular arrangement. On the other hand, certain studies have employed “Parallel-patterned” system. These models are advantageous as they allow variations in blood vessel geometry [55] and observation of smooth muscle cell migration [56]. Su et al. examined the early atherogenic characteristics of endothelial cells, smooth muscle cells, and monocytes introducing the oxidized LDL and inflammatory cytokines [56]. They further assessed the athero-protective impacts of vitamin D and metformin within the disease model. Cho et al. designed an artery model with enhanced morphological similarity by circumferential wrinkles on a PDMS substrate [57, 58]. This aligned the smooth muscle cells and facilitated the axial alignment of endothelial cells during perfusion. Other research has utilized the "Cylindrical blood vessel" system, integrating smooth muscle cells into the extracellular matrix [59, 60]. Specifically, Zhang et al. evaluated vasoactivity, monocyte adhesion, and vessel permeability in their artery model, depending on the presence of enzyme-modified LDL [60]. They also tested the effects of lovastatin and the P2Y11 inhibitor NF157 within this model. Using a distinct approach, Gu et al. developed an arterial model by layering smooth muscle cells, extracellular matrix, and endothelial cells on a stretchable microfluidic device, to observe the circumferential stretch induced differentiation and disease condition [61]. Some studies, while not involving co-cultures of smooth muscle cells, also investigated the reactions of endothelial cells under various flow conditions. They simulated disturbed flow by utilizing bifurcating geometries [62], ridge-shaped impediments [63], and oscillatory perfusion [64].
4.2.2 Neurovascular Model
Numerous studies have employed MPS to resemble the blood–brain barrier (BBB) and associated neurovascular diseases, and those can be categorized based on the employed cells and MPS type (Fig. 3B, Table 1). In basic models, researchers incorporate endothelial cells and astrocytes; more complex studies also include stromal cells, microglia, and neurons.
Applying these BBB models, people has simulated pathologic conditions such as ischemic stroke [65] and Alzheimer's disease, by adapting patient-derived cells [66] or high glucose concentrations [67]. Additionally, studies have also explored the impacts of inflammatory cytokines [68, 69], particulate matter [70, 71], and neurotoxic substances [72, 73] on the neurovascular system. For instance, Lyu et al. emulated an ischemic condition by culturing their neurovascular model under nutrient-deficient and low-oxygen conditions [65]. Various studies have also examined the therapeutic effects of different stem cells on damaged neurovascular environment, focusing on endogenous recovery. Shin et al. highlighted pathological characteristics in an Alzheimer's disease model, including the β-amyloid (Aβ) peptides deposition in the endothelium and the therapeutic effects of Etodolac on barrier restoration [66]. Jang et al. examined the relationship between hyperglycemia, Alzheimer’s disease, and sirtuin 1 (SIRT1) using their BBB model [67]. They further observed that glucose level restoration and the resveratrol (a SIRT1 activator) counteracted the neurodegenerative process. Yang et al. reported the anti-inflammatory effects of omega-3 fatty acids in a BBB model exposed to IL-1β [69]. Matthiesen et al. [73] explored BBB disruption by linsidomine and the protective effects of the antioxidant N-acetylcysteine amide. Seo et al. [74] demonstrated that diesel exhaust particle (DEP)-induced neurodegeneration stemmed from a cascade through granulocyte–macrophage colony-stimulating factor secreted by endothelial cells. This circumstance, in turn, activated microglia, which then produced reactive oxygen species (ROS). They proposed that disrupting the individual steps could mitigated the detrimental effects of this cascade.
4.2.3 Retinal Model
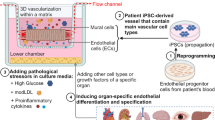
There have been relatively limited studies using MPS to simulate the inner blood–retinal barriers (iBRBs). One study employed a microfluidic device comprising channels with microgrooves to facilitate intercellular interaction [79]. This system also integrated electrodes to measure the barrier function by transepithelial electrical resistance (TEER) assay. Within each channel, primary human retinal endothelial cells, SH-SY5Y cells (a neuroblastoma cell line), and ARPE-19 cells (an RPE cell line) were cultured to construct the iBRB. The model's barrier function was assessed using permeability assays, TEER measurements, and ZO-1 expression evaluation. Another research utilized a “parallel-patterned” model and applied perfusion to the endothelium [80]. The barrier integrity of blood vessels was also evaluated using permeability tests, depending on the endothelial cell types, the presence of perfusion, various cytokines, and their inhibitors.
Additionally, there is a body of work focused on modeling the outer blood–retinal barrier (oBRB) and its associated diseases (Fig. 3C). MPS of oBRB and choroidal blood vessels can be grouped into two categories: oBRB models that culture RPE and EC in a "multi-layered" system [81,82,83,84,85] and the "self-organized μVNs" model [86,87,88,89]. Several studies have resembled choroidal neovascularization via conditions believed to induce wet AMD, such as mechanical stress [84], hypoxia [85, 86], or VEGF [88]. Notably, Chung et al. introduced a drug into their disease model, which successfully inhibited choroidal vessel angiogenesis [88]. Another investigation observed the reduced barrier function of the oBRB and degeneration of choroidal vessels under diabetic conditions [89]. Further studies have induced inflammation in the choroidal layer using biochemicals like CCL19 [82] or hydrogen peroxide [83]. Notably, Cipriano et al. examined immune cell recruitment in their model and evaluated the efficacy of the immunosuppressive drug Cyclosporine (CsA) [82].
4.2.4 Renal Model
The kidney-on-a-chip has been designed utilizing both the “multi-layered” and “parallel-patterned” models (Fig. 3D). These models have successfully recapitulated essential kidney components, such as the Bowman’s capsule-glomerulus, the proximal tubule-peritubular capillary, and the combined nephron structure. A summary of kidney models, based on MPS, is provided in the accompanying table (Table 2).
The relevant literature examined the nephrotoxicity triggered by various compounds including anti-cancer drugs [90,91,92,93,94], antibiotics [94, 95], and other medications [94, 96]. Furthermore, studies have also explored the nephron dysfunction arising from pathologic conditions, such as diabetes mellitus [95, 97] and protein overloading [98].
Specifically, Yin et al. assessed the toxicity of agents like cisplatin, gentamycin, and cyclosporin A within their peritubular capillary model [94]. Notably, they elucidated the protective effect of cimetidine against cisplatin-induced nephrotoxicity. Another study by Petrosyan et al. documented glomerular dysfunction induced by the puromycin aminonucleoside, serum from membranous nephropathy patients, and high glucose concentrations [95]. In addition, they demonstrated that the introduction of α-melanocyte-stimulating hormone reduce albumin leakage of disease model, thereby proving its potential as a diagnostic and drug screening platform. Liu et al. resembled proteinuria in their model through an excessive protein load into the renal tubule, leading to trans-differentiation and apoptosis of endothelial cells and pericytes [98]. Furthermore, they discovered that inhibiting FUT8 could mitigate these deleterious effects associated with proteinuria.
5 Conclusion
There has been significant growth and improvement in in vitro vascular models due to their ease of experimental manipulation and enhanced in vivo resemblance. In particular, these platforms have diversified across various tissues by coculturing different cells and inventing distinct fabrication methods. While these models have broadened our understanding of numerous diseases and organs associated with the vascular system, several aspects remain open to improvement.
First, the fabrication and experimental processes of MPS have not been standardized. It necessitates a certain level of proficiency among researchers attempting to utilize these models. As demonstrated in this review, particularly for the last 10 years, MPS has evolved into extremely varied structures. This diversification has expanded the range of tissues that can be mimicked using MPS; however, the variability of design could present obstacles for users who are trying to simply adapt these systems rather than creating entirely new structures. This issue gains greater significance in biological industries, which demand high reproducibility compared to pioneering biological research. In response, recently emerged models have improved robustness and user convenience, and some have even achieved commercialization. We anticipate that the vascular models discussed in this review can undergo similar improvements, which will further enhance their economic value and utility.
Second, there is still room to incorporate a broader range of analytical tools, and recent MPS studies have already been adapting. Conventionally, MPS has concentrated on modeling the environment and cell differentiation of the target tissue, frequently relying on image-based validations such as immunostaining and cell morphology measurements. The small sample volume of MPS often limits the use of conventional biological assays, although it can be advantageous in fabrication perspective. However, current studies actively utilize a range of tools, from relatively conventional ones like RT-PCR, western blot, and ELISA to more advanced techniques like cytokine arrays, RNA sequencing, and even atomic force microscopy (AFM). Looking forward, there is an expectation that the integration of emerging AI technologies can facilitate exploration into areas that have not been elucidated so far.
In summary, there have been substantial recent advancements in in vitro vascular models. They have successfully achieved a balance between the complexity of the animal model and the oversimplified nature of the 2D culture model, while also minimized a genetic discrepancy using human-derived cells. The ability to emulate various human structures, including functional vascular systems, holds promise for significantly expanding our understanding of numerous diseases.
Data availability
Data sharing is not applicable to this article as no new data were created in this study.
References
Daneman, R., Prat, A.: The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 7, a020412 (2015)
Nagy, J.A., Chang, S.H., Dvorak, A.M., Dvorak, H.F.: Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 100, 865–869 (2009)
Bennis, A., et al.: Comparison of mouse and human retinal pigment epithelium gene expression profiles: potential implications for age-related macular degeneration. PLoS ONE 10, e0141597 (2015)
Hay, M., Thomas, D.W., Craighead, J.L., Economides, C., Rosenthal, J.: Clinical development success rates for investigational drugs. Nat. Biotechnol. 32, 40–51 (2014)
Han, J., Kang, U., Moon, E.-Y., Yoo, H., Gweon, B.: Imaging technologies for microfluidic biochips. BioChip J. 16, 255–269 (2022)
Kang, S.-M.: Recent advances in microfluidic-based microphysiological systems. BioChip J. 16, 13–26 (2022)
Heydari, Z., et al.: Organoids: a novel modality in disease modeling. Bio-des. Manuf. 4, 689–716 (2021)
Kim, J., Koo, B.-K., Knoblich, J.A.: Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020)
Savoji, H., et al.: Cardiovascular disease models: a game changing paradigm in drug discovery and screening. Biomaterials 198, 3–26 (2019)
Volland, S., Esteve-Rudd, J., Hoo, J., Yee, C., Williams, D.S.: A comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS ONE 10, e0125631 (2015)
Van Norman, G.A.: Limitations of animal studies for predicting toxicity in clinical trials: is it time to rethink our current approach? JACC: Basic Transl. Sci. 4, 845–854 (2019)
Ma, C., Peng, Y., Li, H., Chen, W.: Organ-on-a-chip: a new paradigm for drug development. Trends Pharmacol. Sci. 42, 119–133 (2021)
Portillo-Lara, R., Annabi, N.: Microengineered cancer-on-a-chip platforms to study the metastatic microenvironment. Lab Chip 16, 4063–4081 (2016)
Li, X., Valadez, A.V., Zuo, P., Nie, Z.: Microfluidic 3D cell culture: potential application for tissue-based bioassays. Bioanalysis 4, 1509–1525 (2012)
Maurissen, T.L., et al.: Microphysiological neurovascular barriers to model the inner retinal microvasculature. J. Pers. Med. 12, 148 (2022)
Offeddu, G.S., et al.: An on-chip model of protein paracellular and transcellular permeability in the microcirculation. Biomaterials 212, 115–125 (2019)
Caliari, S.R., Burdick, J.A.: A practical guide to hydrogels for cell culture. Nat. Methods 13, 405–414 (2016)
Wörsdörfer, P., et al.: Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 9, 15663 (2019)
Park, W., Lee, J.-S., Gao, G., Kim, B.S., Cho, D.-W.: 3D bioprinted multilayered cerebrovascular conduits to study cancer extravasation mechanism related with vascular geometry. Nat. Commun. 14, 7696 (2023)
Wan, Z., et al.: New strategy for promoting vascularization in tumor spheroids in a microfluidic assay. Adv. Healthcare Mater. 12, 2201784 (2023)
Wimmer, R.A., et al.: Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510 (2019)
Lewis-Israeli, Y.R., et al.: Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 12, 5142 (2021)
Homan, K.A., et al.: Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262 (2019)
Chang, W.G., Niklason, L.E.: A short discourse on vascular tissue engineering. npj Regen. Med. 2, 7 (2017)
Cao, X., Maharjan, S., Ashfaq, R., Shin, J., Zhang, Y.S.: Bioprinting of small-diameter blood vessels. Engineering 7, 832–844 (2021)
Devillard, C.D., Marquette, C.A.: Vascular tissue engineering: challenges and requirements for an ideal large scale blood vessel. Front. Bioeng. Biotechnol. (2021). https://doi.org/10.3389/fbioe.2021.721843
Hwang, D.G., Choi, Y.-M., Jang, J.: 3D bioprinting-based vascularized tissue models mimicking tissue-specific architecture and pathophysiology for in vitro studies. Front. Bioeng. Biotechnol. (2021). https://doi.org/10.3389/fbioe.2021.685507
Oh, H., et al.: Fabrication of hydrogel microchannels using aqueous two-phase printing for 3D blood brain barrier. BioChip J. 17, 369–383 (2023)
Xu, C., Chai, W., Huang, Y., Markwald, R.R.: Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol. Bioeng. 109, 3152–3160 (2012)
Xiong, R., Zhang, Z., Chai, W., Huang, Y., Chrisey, D.B.: Freeform drop-on-demand laser printing of 3D alginate and cellular constructs. Biofabrication 7, 045011 (2015)
Gao, G., Park, J.Y., Kim, B.S., Jang, J., Cho, D.W.: Coaxial cell printing of freestanding, perfusable, and functional in vitro vascular models for recapitulation of native vascular endothelium pathophysiology. Adv. Healthc. Mater. 7, e1801102 (2018)
Gold, K.A., et al.: 3D bioprinted multicellular vascular models. Adv. Healthc. Mater. 10, 2101141 (2021)
Jia, W., et al.: Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 106, 58–68 (2016)
Wu, Z., et al.: Microfluidic printing of tunable hollow microfibers for vascular tissue engineering. Adv. Mater. Technol. 6, 2000683 (2021)
Gao, G., et al.: Construction of a novel in vitro atherosclerotic model from geometry-tunable artery equivalents engineered via in-bath coaxial cell printing. Adv. Func. Mater. 31, 2008878 (2021)
Guillemot, F., et al.: High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 6, 2494–2500 (2009)
Gao, Q., et al.: 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater. Sci. Eng. 3, 399–408 (2017)
Dai, X., et al.: Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci. Rep. 7, 1457 (2017)
Gao, Q., He, Y., Fu, J.Z., Liu, A., Ma, L.: Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 61, 203–215 (2015)
Gao, G., et al.: Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing. Appl. Phys. Rev. 6, 041402 (2019)
Wu, Z., et al.: Microfluidic printing of tunable hollow microfibers for vascular tissue engineering. Adv. Mater. Technol. (2021). https://doi.org/10.1002/admt.202000683
Kim, S., Kim, W., Lim, S., Jeon, J.S.: Vasculature-on-a-chip for in vitro disease models. Bioengineering (2017). https://doi.org/10.3390/bioengineering4010008
Ahn, J., Kim, J., Jeon, J.S., Jang, Y.J.: A microfluidic stretch system upregulates resistance exercise-related pathway. BioChip J. 16, 158–167 (2022)
Bhatia, S.N., Ingber, D.E.: Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014)
Zhang, M., et al.: Biomimetic human disease model of SARS-CoV-2-induced lung injury and immune responses on organ chip system. Adv. Sci. 8, 2002928 (2021)
Kim, S., et al.: 3D vascularized microphysiological system for investigation of tumor-endothelial crosstalk in anti-cancer drug resistance. Biofabrication 15, 045016 (2023)
Bae, J., Kim, M.-H., Han, S., Park, S.: Development of tumor-vasculature interaction on chip mimicking vessel co-option of glioblastoma. BioChip J. 17, 77–84 (2023)
Kwak, T.J., Lee, E.: In vitro modeling of solid tumor interactions with perfused blood vessels. Sci Rep 10, 20142 (2020)
Zhang, S., et al.: Interstitial flow promotes the formation of functional microvascular networks in vitro through upregulation of matrix metalloproteinase-2. Adv. Funct. Mater. 32, 2206767 (2022)
Lee, W., et al.: Machine learning-aided three-dimensional morphological quantification of angiogenic vasculature in the multiculture microfluidic platform. BioChip J. 17, 357–368 (2023)
Liu, J., et al.: Synthetic extracellular matrices with tailored adhesiveness and degradability support lumen formation during angiogenic sprouting. Nat. Commun. 12, 3402 (2021)
van Engeland, N.C., et al.: A biomimetic microfluidic model to study signalling between endothelial and vascular smooth muscle cells under hemodynamic conditions. Lab Chip 18, 1607–1620 (2018)
Ersland, E., et al.: Human vascular wall microfluidic model for preclinical evaluation of drug-induced vascular injury. Tissue Eng. Part C Methods 28, 83–92 (2022)
Youn, J., Han, H., Park, S.M., Kim, D.S.: Arterial internal elastic lamina-inspired membrane for providing biochemical and structural cues in developing artery-on-a-chip. ACS Macro Lett. 10, 1398–1403 (2021)
Menon, N.V., Tay, H.M., Wee, S.N., Li, K.H.H., Hou, H.W.: Micro-engineered perfusable 3D vasculatures for cardiovascular diseases. Lab Chip 17, 2960–2968 (2017)
Su, C., et al.: A novel human arterial wall-on-a-chip to study endothelial inflammation and vascular smooth muscle cell migration in early atherosclerosis. Lab Chip 21, 2359–2371 (2021)
Cho, M., Park, J.-K.: Fabrication of a perfusable 3D in vitro artery-mimicking multichannel system for artery disease models. ACS Biomater. Sci. Eng. 6, 5326–5336 (2020)
Cho, M., Park, J.-K.: Modular 3D in vitro artery-mimicking multichannel system for recapitulating vascular stenosis and inflammation. Micromachines 12, 1528 (2021)
Tan, A., Fujisawa, K., Yukawa, Y., Matsunaga, Y.: Bottom-up fabrication of artery-mimicking tubular co-cultures in collagen-based microchannel scaffolds. Biomater. Sci. 4, 1503–1514 (2016)
Zhang, X., et al.: Modeling early stage atherosclerosis in a primary human vascular microphysiological system. Nat. Commun. 11, 5426 (2020)
Gu, X., Xie, S., Hong, D., Ding, Y.: An in vitro model of foam cell formation induced by a stretchable microfluidic device. Sci. Rep. 9, 1–11 (2019)
Akbari, E., Spychalski, G.B., Rangharajan, K.K., Prakash, S., Song, J.W.: Flow dynamics control endothelial permeability in a microfluidic vessel bifurcation model. Lab Chip 18, 1084–1093 (2018)
Tovar-Lopez, F., et al.: A microfluidic system for studying the effects of disturbed flow on endothelial cells. Front. Bioeng. Biotechnol. 7, 81 (2019)
Sei, Y.J., Ahn, S.I., Virtue, T., Kim, T., Kim, Y.: Detection of frequency-dependent endothelial response to oscillatory shear stress using a microfluidic transcellular monitor. Sci. Rep. 7, 1–8 (2017)
Lyu, Z., et al.: A neurovascular-unit-on-a-chip for the evaluation of the restorative potential of stem cell therapies for ischaemic stroke. Nat. Biomed. Eng. 5, 847–863 (2021)
Shin, Y., et al.: Blood-brain barrier dysfunction in a 3D in vitro model of Alzheimer’s disease. Adv Sci (Weinh) 6, 1900962 (2019)
Jang, M., Choi, N., Kim, H.N.: Hyperglycemic neurovasculature-on-a-chip to study the effect of SIRT1-targeted therapy for the type 3 diabetes “Alzheimer’s disease.” Adv. Sci. 9, 2201882 (2022)
Pediaditakis, I., et al.: A microengineered brain-chip to model neuroinflammation in humans. iScience 25, 104813 (2022)
Yang, T., et al.: Protective effects of omega-3 fatty acids in a blood–brain barrier-on-chip model and on postoperative delirium-like behaviour in mice. Br. J. Anaesth. 130, e370–e380 (2023)
Seo, S., et al.: Neuro-glia-vascular-on-a-chip system to assess aggravated neurodegeneration via brain endothelial cells upon exposure to diesel exhaust particles. Adv. Funct. Mater. 33, 2210123 (2023)
Li, Y., et al.: Study of the neurotoxicity of indoor airborne nanoparticles based on a 3D human blood-brain barrier chip. Environ. Int. 143, 105598 (2020)
Koo, Y., Hawkins, B.T., Yun, Y.: Three-dimensional (3D) tetra-culture brain on chip platform for organophosphate toxicity screening. Sci. Rep. 8, 2841 (2018)
Matthiesen, I., Voulgaris, D., Nikolakopoulou, P., Winkler, T.E., Herland, A.: Continuous monitoring reveals protective effects of N-acetylcysteine amide on an isogenic microphysiological model of the neurovascular unit. Small 17, 2101785 (2021)
Seo, S., Nah, S.Y., Lee, K., Choi, N., Kim, H.N.: Triculture model of in vitro BBB and its application to study BBB-associated chemosensitivity and drug delivery in glioblastoma. Adv. Funct. Mater. 32, 2106860 (2022)
Campisi, M., et al.: 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180, 117–129 (2018)
Kim, S., et al.: Human bone marrow-derived mesenchymal stem cells play a role as a vascular pericyte in the reconstruction of human BBB on the angiogenesis microfluidic chip. Biomaterials 279, 121210 (2021)
Lee, S., Chung, M., Lee, S.R., Jeon, N.L.: 3D brain angiogenesis model to reconstitute functional human blood-brain barrier in vitro. Biotechnol. Bioeng. 117, 748–762 (2020)
Adriani, G., Ma, D., Pavesi, A., Kamm, R.D., Goh, E.L.: A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 17, 448–459 (2017)
Yeste, J., et al.: A compartmentalized microfluidic chip with crisscross microgrooves and electrophysiological electrodes for modeling the blood–retinal barrier. Lab Chip 18, 95–105 (2018)
Ragelle, H., et al.: Human retinal microvasculature-on-a-chip for drug discovery. Adv. Healthc. Mater. 9, 2001531 (2020)
Chen, L.-J., Raut, B., Nagai, N., Abe, T., Kaji, H.: Prototyping a versatile two-layer multi-channel microfluidic device for direct-contact cell-vessel co-culture. Micromachines 11, 79 (2020)
Cipriano, M., et al.: Human immunocompetent choroid-on-chip: a novel tool for studying ocular effects of biological drugs. Commun. Biol. 5, 52 (2022)
Arık, Y.B., et al.: Microfluidic organ-on-a-chip model of the outer blood-retinal barrier with clinically relevant read-outs for tissue permeability and vascular structure. Lab Chip 21, 272–283 (2021)
Farjood, F., Vargis, E.: Novel devices for studying acute and chronic mechanical stress in retinal pigment epithelial cells. Lab Chip 18, 3413–3424 (2018)
Chen, L.J., et al.: Microfluidic co-cultures of retinal pigment epithelial cells and vascular endothelial cells to investigate choroidal angiogenesis. Sci. Rep. 7, 3538 (2017)
Lee, S., Kim, S., Jeon, J.S.: Microfluidic outer blood-retinal barrier model for inducing wet age-related macular degeneration by hypoxic stress. Lab Chip 22, 4359–4368 (2022)
Paek, J., et al.: Microphysiological engineering of self-assembled and perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS Nano 13, 7627–7643 (2019)
Chung, M., et al.: Wet-AMD on a chip: modeling outer blood-retinal barrier in vitro. Adv. Healthc. Mater. (2018). https://doi.org/10.1002/adhm.201700028
Nam, U., Lee, S., Jeon, J.S.: Generation of a 3D outer blood-retinal barrier with advanced choriocapillaris and its application in diabetic retinopathy in a microphysiological system. ACS Biomater. Sci. Eng. 9, 4929–4939 (2023)
Qu, Y., et al.: A nephron model for study of drug-induced acute kidney injury and assessment of drug-induced nephrotoxicity. Biomaterials 155, 41–53 (2018)
Zhang, S.Y., Mahler, G.J.: A glomerulus and proximal tubule microphysiological system simulating renal filtration, reabsorption, secretion, and toxicity. Lab Chip (2023). https://doi.org/10.1039/D2LC00887D
Roye, Y., et al.: A personalized glomerulus chip engineered from stem cell-derived epithelium and vascular endothelium. Micromachines 12, 967 (2021)
Musah, S., et al.: Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 1, 0069 (2017)
Yin, L., et al.: Efficient drug screening and nephrotoxicity assessment on co-culture microfluidic kidney chip. Sci. Rep. 10, 6568 (2020)
Petrosyan, A., et al.: A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat. Commun. 10, 3656 (2019)
Vedula, E.M., Alonso, J.L., Arnaout, M.A., Charest, J.L.: A microfluidic renal proximal tubule with active reabsorptive function. PLoS ONE 12, e0184330 (2017)
Wang, L., et al.: A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip 17, 1749–1760 (2017)
Liu, A., et al.: Core fucosylation involvement in the paracrine regulation of proteinuria-induced renal interstitial fibrosis evaluated with the use of a microfluidic chip. Acta Biomater. 142, 99–112 (2022)
Imaoka, T., et al.: Bridging the gap between in silico and in vivo by modeling opioid disposition in a kidney proximal tubule microphysiological system. Sci. Rep. 11, 21356 (2021)
Chapron, A., et al.: An improved vascularized, dual-channel microphysiological system facilitates modeling of proximal tubular solute secretion. ACS Pharmacol. Transl. Sci. 3, 496–508 (2020)
Menéndez, A.B.-C., et al.: Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci. Rep. 12, 20699 (2022)
Zhang, S.Y., Mahler, G.J.: Modelling renal filtration and reabsorption processes in a human glomerulus and proximal tubule microphysiological system. Micromachines 12, 983 (2021)
Sakolish, C.M., Mahler, G.J.: A novel microfluidic device to model the human proximal tubule and glomerulus. RSC Adv. 7, 4216–4225 (2017)
Acknowledgements
This study was funded by the National Research Foundation of Korea (2020R1A5A8018367) and the Brain Korea 21 Plus program.
Funding
Open Access funding enabled and organized by KAIST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nam, U., Lee, S., Ahmad, A. et al. Microphysiological Systems as Organ-Specific In Vitro Vascular Models for Disease Modeling. BioChip J (2024). https://doi.org/10.1007/s13206-024-00152-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13206-024-00152-4